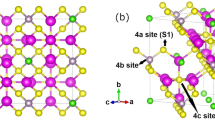
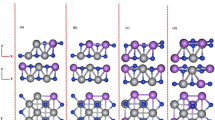
Abstract
Diffusion of Li in Li x Sn alloys was investigated using a density functional theory in order to fully understand the lithiation process in these types of Li ion batteries. Variation of the calculated open-circuit voltages of the Li x Sn alloys was found to agree well with experimental results. Diffusion coefficients of the Li in the Li x Sn alloys were calculated to be in the range between 6.6 × 10−8 and 5.6 × 10−7 cm2 s−1 at room temperature, which is within the range between 8.0 × 10−8 and 5.9 × 10−7 cm2 s−1 obtained from the experimental measurement.




Similar content being viewed by others
References
Zhang H, Sun X, Zhang X, Lin H, Wang K, Ma Y (2015) High-capacity nanocarbon anodes for lithium-ion batteries. J Alloy Comp 622:783–788
Eom K, Jung J, Lee JT, Lair V, Joshi T, Lee SW, Lin Z, Fuller TF (2015) Improved stability of nano-Sn electrode with high-quality nano-SEI formation for lithium ion battery. Nano Energy 12:314–321
Kiziltas-Yavuz N, Bhaskar A, Dixon D, Yavuz M, Nikolowski K, Lu L, Eichel R-A, Ehrenberg H (2014) Improving the rate capability of high voltage lithium-ion battery cathode material LiNi0.5Mn1.5O4 by ruthenium doping. J Power Sources 267:533–541
Zhang W-J (2011) A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J Power Sources 196:13–24
Kim R, Nam D, Kwon H (2010) Electrochemical performance of a tin electrodeposit with a multi-layered structure for Li-ion batteries. J Power Sources 195:5067–5070
Hu RZ, Zeng MQ, Zhu M (2009) Cyclic durable high-capacity Sn/Cu6Sn5 composite thin film anodes for lithium ion batteries prepared by electron-beam evaporation deposition. Electrochim Acta 54:2843–2850
Zhao LZ, Hu SJ, Ru Q, Li WS, Hou XH, Zeng RH, Lu DS (2008) Effects of graphite on electrochemical performance of Sn/C composite thin film anodes. J Power Sources 184:481–484
Hu R, Liu H, Zeng M, Liu J, Zhu M (2012) Progress on Sn-based thin-film anode materials for lithium-ion batteries. Chin Sci Bull 57:4119–4130
Chou C-Y, Kim H, Hwang GS (2011) A comparative first-principles study of the structure, energetics, and properties of Li-M (M = Si, Ge, Sn) alloys. J Phys Chem C 115:20018–20026
Robert F, Lippens PE, Fourcade R, Jumas J-C, Gillot F, Morcrette M, Tarascon J-M (2006) Mechanosynthesis and characterisation of the Li–Sn system. Hyperfine Interact 167:797–801
Nithyadharseni P, Reddy MV, Nalini B, Kalpana M, Chowdari BVR (2015) Sn-based intermetallic alloy anode materials for the application of lithium ion batteries. Electrochim Acta 161:261–268
Nithya C, Gopukumar S (2013) Reduced graphite oxide/nano Sn: a superior composite anode material for rechargeable lithium-ion batteries. ChemSusChem 6:898–904
Zhang HK, Song HH, Chen XH, Zhou JS (2012) Enhanced lithium ion storage property of Sn nanoparticles: the confinement effect of few-walled carbon nanotubes. J Phys Chem C 116:22774–22779
Li Y, Wu J, Chopra N (2015) Nano-carbon-based hybrids and heterostructures: progress in growth and application for lithium-ion batteries. J Mater Sci 50:7843–7865
Jiang H, Ge Y, Fu K, Lu Y, Chen C, Zhu J, Dirican M, Zhang X (2015) Centrifugally-spun tin-containing carbon nanofibers as anode material for lithium-ion batteries. J Mater Sci 50:1094–1102
Qiao H, Zheng Z, Zhang L, Xiao L (2008) SnO(2)@C core-shell spheres: synthesis, characterization, and performance in reversible Li-ion storage. J Mater Sci 43:2778–2784
Szabo DV, Kilibarda G, Schlabach S, Trouillet V, Bruns M (2012) Structural and chemical characterization of SnO2-based nanoparticles as electrode material in Li-ion batteries. J Mater Sci 47:4383–4391
Wang XL, Han WQ, Chen J, Graetz J (2010) Single-crystal intermetallic M-Sn (M = Fe, Cu Co, Ni) nanospheres as negative electrodes for lithium-ion batteries. ACS Appl Mater Interface 2:1548–1551
Zhang PP, Ma ZS, Wang Y, Zou YL, Lei WX, Pan Y, Lu CS (2015) A first principles study of the mechanical properties of Li–Sn alloys. Rsc Adv 5:36022–36029
Wen CJ, Huggins RA (1981) Chemical diffusion in intermediate phases in the lithium-silicon system. J Solid State Chem 37:271–278
Nimon ES, Churikov AV (1996) Electrochemical behaviour of Li–Sn, Li–Cd and Li–Sn–Cd alloys in propylene carbonate solution. Electrochim Acta 41:1455–1464
Winter M, Besenhard JO (1999) Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim Acta 45:31–50
Machill S, Rahner D (1995) In-situ electrochemical characterization of lithium-alloying materials for rechargeable anodes in lithium batteries. J Power Sources 54:428–432
Wang J, Raistrick ID, Huggins RA (1986) Behavior of some binary lithium alloys as negative electrodes in organic solvent-based electrolytes. J Electrochem Soc 133:457–460
Courtney IA, Tse JS, Mao O, Hafner J, Dahn JR (1998) Ab initio calculation of the lithium-tin voltage profile. Phys Rev B 58:15583–15588
Jianjian Shi WS, Jin Wei, Yin Guangqiang (2015) Diffusion of lithium in α-Sn and β-Sn as anode materials for lithium ion batteries. Inter J Electrochem Sci Technol Adv Mater 10:4793–4800
Genser O, Hafner J (2001) Structure and bonding in crystalline and molten Li–Sn alloys: a first-principles density-functional study. Phys Rev B 63(14):144204
Zhang T, Fu LJ, Gao J, Wu YP, Holze R, Wu HQ (2007) Nanosized tin anode prepared by laser-induced vapor deposition for lithium ion battery. J Power Sources 174:770–773
Xie J, Imanishi N, Hirano A, Takeda Y, Yamamoto O, Zhao XB, Cao GS (2010) Li-ion diffusion behavior in Sn, SnO and SnO2 thin films studied by galvanostatic intermittent titration technique. Solid State Ionics 181:1611–1615
Soler JM, Artacho E, Gale JD, Garcia A, Junquera J, Ordejon P, Sanchez-Portal D (2002) The SIESTA method for ab initio order-N materials simulation. J Phys-Condens Mat 14:2745–2779
Legrain F, Malyi O, Manzhos S (2015) Insertion energetics of lithium, sodium, and magnesium in crystalline and amorphous titanium dioxide: a comparative first-principles study. J Power Sources 278:197–202
Legrain F, Malyi OI, Manzhos S (2014) Comparative computational study of the energetics of Li, Na, and Mg storage in amorphous and crystalline silicon. Comp Mater Sci 94:214–217
Kim H, Chou C-Y, Ekerdt JG, Hwang GS (2011) Structure and properties of Li–Si alloys: a first-principles study. J Phys Chem C 115:2514–2521
Janz GJ, Kerbs U, Siegenthaler H (1972) Molten salts: nitrates, nitrites, and mixtures: electrical conductance, density, viscosity, and surface tension data. J Phys Chem Ref Data 1:581–746
Manthiram A, Chemelewski K, Lee ES (2014) A perspective on the high-voltage LiMn1.5Ni0.5O4 spinel cathode for lithium-ion batteries. Energy Environ Sci 7:1339–1350
Aydinol MK, Kohan AF, Ceder G, Cho K, Joannopoulos J (1997) Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Phys Rev B 56:1354–1365
Winter M, Besenhard JO (1999) Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim Acta 45:31–50
Anani A, Crouch-Baker S, Huggins RA (1987) Kinetic and thermodynamic electrode materials at ambient temperature. J Electrochem Soc 134:3098–3102
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (11474047). Funding support from the UoA and CAPEX funding from Northumbria University at Newcastle upon Tyne, Royal academy of Engineering UK-Research Exchange with China and India is acknowledged. This work was carried out at National Supercomputer Center in Tianjin, and the calculations were performed on TianHe-1(A).
Funding
This study was funded by National Natural Science Foundation of China (11474047) and Royal academy of Engineering UK-Research Exchange with China and India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shi, J., Wang, Z. & Fu, Y.Q. Density functional theory study of diffusion of lithium in Li–Sn alloys. J Mater Sci 51, 3271–3276 (2016). https://doi.org/10.1007/s10853-015-9639-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9639-z