Abstract
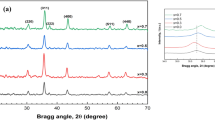
Potassium dihydrogen phosphate KH2PO4 (KDP) single crystals, both pure and with incorporated titanium dioxide TiO2 nanoparticles, are ferroelectric materials with dipole structure used in nonlinear optics, optoelectronics, and acoustooptics. To date, a comprehensive analysis of structural features and defect states in the KDP and KDP/TiO2 matrix is absent in literature data. Pure KH2PO4 (KDP) single crystals and KDP/TiO2 crystals with different TiO2 nanoparticles with anatase structure, synthesized by the sulfate and chloride methods, and with η-TiO2 structure, prepared by the sulfate method, grown by the temperature lowering method and cut from the pyramidal and prismatic growth sectors, have been investigated by the X-ray diffraction methods and by EDX spectroscopy. According to the energy-dispersive X-ray microanalysis, the sulfur content is higher in the sample with η-TiO2 and in the KDP/η-TiO2 crystal if compared with the samples with anatase and KDP/TiO2 (anatase). It was revealed that the sulfur content is higher in the prismatic growth sector if compared with the pyramidal one. Based on results of the X-ray single-crystal diffraction analysis, it is possible to assume the substitution of \( \text{PO}_{4}^{3 - } \) tetrahedra by \( \text{SO}_{4}^{2 - } \) ones in the KDP/TiO2 structures, which is greater in KDP/η-TiO2. The general composition of the KDP/η-TiO2 crystal from the prismatic growth sector with the highest titanium content can be written as \( (\text{K}_{0.950(1)} \square_{0.050} )\text{Ti}_{0.052\left( 2 \right)i} \left( {\text{H}_{2 - x}^{1 + } \square_{x} } \right)\left[ {\left( {\text{PO}_{4} } \right)_{y}^{3 - } \left( {\text{SO}_{4}^{2 - } } \right)_{1 - y} } \right] (\square \text{ - vacancies}). \) It was first found that the Ti4+ ions occupy the interstitial site (−0.048, 0.045, 0.550) in the KDP crystal matrix. Analysis of frequency-dependant dielectric characteristics revealed that the incorporation of TiO2 nanoparticles reduces the dielectric permittivity values of KDP/TiO2 samples compared with the pure KDP ones. It was found that the KDP/η-TiO2 sample has the smallest value of the dielectric permittivity.





Similar content being viewed by others
References
Eimerl D (1987) Electro-optic, linear, and non-linear optical properties of KDP and its isomorphs. Ferroelectrics 72:95–139. doi:10.1080/00150198708017942
Gayvoronsky VY, Kopylovsky MA, Yatsyna VO, Rostotsky AI, Brodyn MS, Pritula IM (2012) Photoinduced refractive index variation in the KDP single crystals with incorporated TiO2 nanoparticles under CW laser excitation. Ukr J Phys 57(2):159–165
Bacon GE, Pease RS (1953) A neutron diffraction study of dihydrogen phosphate by Fourier synthesis. Proc R Soc A220:397–421
Nelmes RJ, Meyer GM, Tibballs JE (1982) The crystal structure of tetragonal KH2PO4 and KD2PO4 as a function of temperature. J Phys C Solid State Phys 15:59–75. doi:10.1088/0022-3719/15/1/005
Bornarel J (1987) Domains in KH2PO4. Ferroelectrics 71:255–268. doi:10.1080/00150198708224840
Suvorova EI, Klechkovskaya VV (1993) Transmission electron microscopy study of KDP crystals. Ferroelectrics 144:245–253. doi:10.1080/00150199308008650
Ichikawa M, Amasaki D, Gustafsson T, Olovsson I (2001) Structural parameters determining the transition temperature of tetragonal KH2PO4-type crystals. Phys Rev B Condens Matter 64(10):100101. doi:10.1103/PhysRevB.64.100101
Pritula IM, Kolybayeva MI, Salo VI, Puzikov VM (2007) Defects of large-size KDP single crystals and their influence on degradation of the optical properties. Opt Mater 30(1):98–100. doi:10.1016/j.optmat.2006.11.003
Dhanaraj PV, Mathew Santheep K, Rajesh NP (2008) Nucleation studies and characterization of potassium dihydrogen phosphate single crystals with l-arginine monohydrochloride as additive. J Cryst Growth 310(10):2532–2536. doi:10.1016/j.jcrysgro.2007.12.001
Zaitseva NP, De Yoreo JJ, Dehaven MR, Vital RL, Montgomery KE, Richardson M, Atherton LG (1997) Rapid growth of large-scale (40–55 cm) KH2PO4 crystals. J Cryst Growth 180:255–262. doi:10.1016/S0022-0248(97)00223-6
Nakatsuka M, Fujioka K, Kanabe T, Fujita H (1997) Rapid growth over 50 mm/day of water-soluble KDP crystal. J Cryst Growth 171(3):531–537. doi:10.1016/S0022-0248(96)00675-6
Pritula I, Gayvoronsky V, Kopylovsky M, Kolybaeva M, Puzikov V, Kosinova A, Tkachenko V, Tsurikov V, Konstantiniva T, Pogibko V (2008) Growth and characterization of KH2PO4 single crystals doped with TiO2 nanocrystals. Funct Mater 15(3):420–428
Pritula I, Gayvoronsky V, Kolybaeva M et al (2010) Effect of incorporation of titanium dioxide nanocrystals on bulk properties of KDP crystals. Opt Mater 33(4):623–630. doi:10.1016/j.optmat.2010.11.022
Gayvoronsky V, Galas A, Shepelyavyy E et al (2005) Giant nonlinear optical response of nanoporous anatase layers. Appl Phys B 80:97–100. doi:10.1007/s00340-004-1676-2
Gayvoronsky VY, Kopylovsky MA, Vishnyakov EA (2009) Optical and nonlinear optical characterization of nanostructured oxyhydroxide of aluminium. Funct Mater 16(2):136–140
Ganduglia-Pirovano MV, Hofmann A, Sauer J (2007) Oxygen vacancies in transition metal and rare earth oxides: current state of understanding and remaining challenges. Surf Sci Rep 62:219–270. doi:10.1016/j.surfrep.2007.03.002
Kosinova AV, Kolybaeva MI, Bezkrovnaya ON, Tkachenko VF, Grishina EV et al (2014) Structural and mechanical properties of KH2PO4 single crystals with embedded nanoparticles and organic molecules. Cryst Res Technol 49(12):965–974. doi:10.1002/crat.201400285
Obolenskaya LN, Kuz’micheva GM, Savinkina EV, Sadovskaya NV, Zhilkina AV, Prokudina NA, Chernyshev VV (2012) Influence of the conditions of the sulfate method on the characteristics of nanosized anatase-type samples. Russ Chem Bull 61(11):2032–2038. doi:10.1007/s11172-012-0286-0
Ismagilov ZR, Tsikoza LT, Shikina NV, Zarytova VF, Zinoviev VV, Zagrebelnyi SN (2009) Synthesis and stabilization of nano-sized titanium dioxide. Russ Chem Rev 78(9):873–885. doi:10.1070/RC2009v078n09ABEH004082
Zhurov VV, Ivanov SA (1997) PROFIT computer program for processing powder diffraction data on an IBM PC with a graphic user interface. Crystallogr Rep 42(2):239–243
Farrugia LG (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Cryst 32:837–838. doi:10.1107/S0021889899006020
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A 64:112–122. doi:10.1107/S0108767307043930
Müller P, Herbst-Irmer R, Spek AL et al (2006) Crystal structure refinement: a crystallographer’s guide to SHELXL. Oxford University Press, Oxford
Kuzmicheva GM, Gainanova AA, Orekhov AS et al (2014) Peculiarities of the microstructure of a nanoscale modification of η-TiO2. Crystallogr Rep 59(6):916–922. doi:10.1134/S1063774514050101
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32(6):751–767. doi:10.1107/S0567739476001551
Malakhova LF, Furmanova NG, Vilensky AI, Grigorieva MS, Simonov VI, Rudneva EB, Voloshin AE (2009) Structural features of the KH2 PO4: Cr single crystal. Crystallogr Rep 54(2):211–218. doi:10.1134/S1063774509020084
Barrett NT, Lamble GM, Roberts KJ et al (1989) Glancing angle EXAFS investigation of the habit modification of ADP by the incorporation of iron. J Cryst Growth 94:689–696. doi:10.1016/0022-0248(89)90093-6
Eremina TA, Kuznetsov VA, Okhrimenko TM, Furmanova NG (1996) The mechanism of incorporating impurities into KDP-group crystals. Crystallogr Rep 41(4):680–684
Eremina TA, Kuznetsov VA, Okhrimenko TM, Furmanova NG, Eremin NN, Urusov VS (1998) Modeling of a defect region in KDP crystals doped with trivalent iron. Crystallogr Rep 43(5):852–857
Acknowledgements
This work was carried out as a part of a state task of the Ministry of Education and Science of Russian Federation (No. 4.745.2014/K; 2014-2016). X-ray studies were fulfilled using a STOE Stadi Vari PILATUS 100 K single-crystal diffractometer purchased by MSU Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuz’micheva, G.M., Timaeva, O.I., Rybakov, V.B. et al. Growth, structure peculiarities, and dielectric properties of ferroelectric KDP/TiO2 single crystals. J Mater Sci 51, 3045–3055 (2016). https://doi.org/10.1007/s10853-015-9615-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9615-7