Abstract
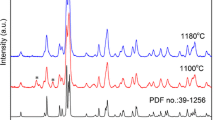
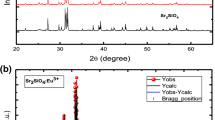
Herein, SrGd2O4:Eu3+ phosphors were synthesized by homogeneous precipitation method followed by combustion process. With an increase in processing temperature from 800 to 1200 °C, these phosphors showed a systematic change from a mixed low crystalline phase to a highly crystalline single phase. The samples, annealed at 1200 °C, were crystallized into orthorhombic phase without any impurities. Microscopic studies revealed the irregular morphology of the obtained phosphor particles having sizes in the range of 0.5–5 μm. UV-excited phosphors exhibited strong red emissions owing to the homogeneous occupation of Eu3+ ions in the host. With the enhancement in Eu3+ concentrations up to 4 mol%, the emission intensity was observed to increase systemically, and decreased above 4 mol% owing to the concentration quenching. The critical distance for the quenching mechanism was calculated to be 17.11 Å suggesting multipolar interactions between Eu3+ ions. The dominant red-to-orange intensity ratios (2.59) and Judd–Ofelt parameters of Eu3+ ions supported the strong covalent nature and site-occupation of higher asymmetry sites of Gd3+ ions. The red colour purity was increased with Eu3+ concentration and obtained to be 95 % for SrGd2O4:4 mol% Eu3+ sample. Efficient red emission with high colour purity and elevated chemical durability indicated the suitability of SrGd2O4:Eu3+ phosphor for display applications.














Similar content being viewed by others
References
Ronda CR, Jüstel T, Nikol H (1998) Rare earth phosphors: fundamentals and applications. J Alloy Compd 275–277:669–676
Arul Antony S, Nagaraja KS, Reddy GLN, Sreedharan OM (2001) A polymeric gel cum auto combustion method for the lower temperature synthesis of SrR2O4 (R = Y, La, Sm, Eu, Gd, Er or Yb). Mater Lett 51:414–419
Raju GSR, Yu JS (2014) Novel orange and reddish-orange color emitting BaGd2O4:Sm3+ nanophosphors by solvothermal reaction for LED and FED applications. Spectrochim Acta A 124:383–388
Zhou L, Shi J, Gong M (2007) Synthesis and luminescent properties of BaGd2O4: Eu3+ phosphor. J Phys Chem Solids 68:1471–1475
Marí B, Singh KC, Sahal M, Khatkar SP, Taxak VB, Kumar M (2011) Characterization and photoluminescence properties of some MLn2(1−x)O4:2xEu3+ or 2xTb3+ systems (M = Ba or Sr, Ln = Gd or La). J Lumin 131:587–591
Sharma KG, Singh NS, Devi YR, Singh NR, Singh SD (2013) Effects of annealing on luminescence of CaWO4:Eu3+ nanoparticles and its thermoluminescence study. J Alloy Compd 556:94–101
Lai Xin, Wei Yanyan, Qin Dan, Zhao Yan, Yun Wu, Gao Daojiang, Bi Jian, Lin Dunmin, Guangliang Xu (2012) Hydrothermal synthesis of Ca(1 − 3x/2)Tb x WO4 microcrystallines and their luminescent properties. Integr Ferroelectr 140:177–186
Gao D, Li Y, Lai X, Wei Y, Bi J, Li Y, Liu M (2011) Fabrication and luminescence properties of Dy3+ doped CaMoO4 powders. Mater Chem Phys 126:391–397
Jeong Y-K, Sohn Y, Kang J-G (2014) Synthesis and characterization of Eu(III)-incorporated silica nanoparticles for application to UV-LED. J Colloid Interface Sci 423:41–47
Kang J-S, Jeong Y-K, Kang J-G, Zhao L, Sohn Y, Pradhan D, Leung KT (2015) Observation of mediated cascade energy transfer in europium-doped ZnO nanowalls by 1,10-phenanthroline. J Phys Chem C 119:2142–2147
Lv L, Wang J, Wang W, Han L (2015) Microstructure control by Y3+ ions doping in CaMoO4:Eu3+: Tunable optical and luminescent performance. J Alloy Compd 635:25–33
Tian Y, Chen B, Hua R, Sun J, Cheng L, Zhong H, Li X, Zhang J, Zheng Y, Tingting Y, Huang L, Yu H (2011) Optical transition, electron-phonon coupling and fluorescent quenching of La2(MoO4)3:Eu3+ phosphor. J Appl Phys 109:053511–053517
Tian Y, Tian B, Chen B, Cui C, Huang P, Wang L, Hua R (2014) Morphological tuning and enhanced luminescence of NaEuF4 nano/submicro-crystals. Appl Surf Sci 313:504–511
Som S, Kunti AK, Kumar V, Kumar V, Dutta S, Chowdhury M, Sharma SK, Terblans JJ, Swart HC (2014) Defect correlated fluorescent quenching and electron phonon coupling in the spectral transition of Eu3+ in CaTiO3 for red emission in display application. J Appl Phys 115:193101–193115
Chowdhury M, Sharma SK (2015) Spectroscopic behaviour of Eu3+ in SnO2 for tunable red emission in solid state lighting devices. RSC Adv 5:51102–51109
Dutta S, Som S, Sharma SK (2015) Excitation spectra and luminescence decay analysis of K+ compensated Dy3+ doped CaMoO4 phosphors. RSC Adv 5:7380–7387
Raju GSR, Pavitra E, Yu JS (2014) Cross-relaxation induced tunable emissions from the Tm3+/Er3+/Eu3+ ions activated BaGd2O4 nanoneedles. Dalton Trans 43:9766–9776
Sun X-Y, Wang W-F, Sun S-Q, Lin L-W, Li D-Y, Zhou L-P (2013) Synthesis and luminescent properties of novel BaGd2O4:Eu3+ scintillating phosphor. Luminescence 28:384–391
Besara T, Lundberg MS, Sun J, Ramirez D, Dong L, Whalen JB, Vasquez R, Herrera JF, Allen MD, Siegrist T (2014) Single crystal synthesis and magnetism of the BaLn2O4 family (Ln = lanthanide). Prog Solid State Chem 42:23–36
Wang D, Wang Y, Wang L (2007) Photoluminescence properties of Sr(Y, Gd)2O4: Eu3+ under VUV excitation. J Lumin 126:135–138
Reddy AA, Das S, Ahmad SS, Babu S, Ferreirab JM, Prakasha GV (2012) Influence of the annealing temperatures on the photoluminescence of KCaBO3:Eu3+ phosphor. RSC Adv 2:8768–8776
Zuoling F, Zhou S, Zhang S (2006) Preparation and optical properties of trivalent europium-doped bulk and nanocrystalline SrY2O4. J Opt Soc Am B 23(9):77–83
Maekawa T, Kurosaki K, Yamanaka S (2007) Thermophysical properties of BaY2O4: a new candidate material for thermal barrier coatings. Mater Lett 61:2303–2306
Zuoling F, Zhou S, Zhang S (2006) Preparation and optical properties of trivalent europium-doped bulk and nanocrystalline SrY2O4. J Opt Soc Am B 23(9):1853–1858
Karunadasa H, Huang Q, Ueland BG, Lynn JW, Schiffer P, Regan KA, Cava RJ (2005) Honeycombs of triangles and magnetic frustration in SrL2O4 (L = Gd, Dy, Ho, Er, Tm, and Yb). Phys Rev B 71:144414–144422
Lakshminarasimhan N, Varadaraju UV (2008) Role of crystallite size on the photoluminescence properties of SrIn2O4:Eu3+ phosphor synthesized by different methods. J Solid State Chem 181:2418–2423
Wang H, Tian L (2011) Luminescence properties of SrIn2O4:Eu3+ incorporated with Gd3+ or Sm3+ ions. J Alloy Compd 509:2659–2662
Sun XY, Liu Y, Liu XL, Cao R-P, Li Y-N, Lin L-W (2014) Substitution of Y3+ for Gd3+ on the luminescent properties of BaGd2O4:Eu3+ scintillating phosphors. Opt Mater 36:1478–1483
Ekambaram S, Patil KC (1997) Synthesis and properties of Eu2+ activated blue phosphors. J Alloy Compd 248:7–12
Li X, Odoom-Wubah T, Chen Z, Zheng B, Huang Jiale (2014) Ethanol-dependent solvothermal synthesis of monodispersed YAG powders with precursor obtained through bubbling ammonia. Ceram Int 40(10):16317–16321
Caglar Y, Ilican S, Caglar M, Yakuphanoglu F, Wu J, Gao K, Lu P, Xue D (2009) Influence of heat treatment on the nanocrystalline structure of ZnO film deposited on p-Si. J Alloy Compd 481:885–889
Sengupta J, Sahoo RK, Bardhan KK, Mukherjee CD (2011) Influence of annealing temperature on the structural, topographical and optical properties of sol–gel derived ZnO thin films. Mater Lett 65:2572–2574
Pal PP, Manam J (2013) Photoluminescence and thermoluminescence studies of Tb3+ doped ZnO nanorods. Mat Sci Eng B 178:400–408
Wang WN, Widiyastuti T, Lenggoro W, Okuyama K (2007) Correlations between crystallite/particle Size and photoluminescence properties of sub micrometer phosphors. Chem Mater 19:1723–1730
Syu J-R, Kumar S, Das S, Lu C-H (2012) Micro emulsion-mediated synthesis and characterization of YBO3:Ce3+ phosphors. J Am Ceram Soc 95(6):1814–1817
Meltzer RS (1999) Dependence of fluorescence lifetimes of Y2O3:Eu3+ nanoparticles on the surrounding medium. Phys Rev B 60(20):14012–14015
Marciniak L, Stefanski M, Tomala R, Hreniak D, Strek W (2015) Size effect in luminescent properties of LiNdP4O12 nanocrystals. Opt Mater 41:17–20
Georgescu S, Cotoi E, Voiculescu AM, Toma O (2008) Effects of particle size on the luminescence of YVO4: Eu nanocrtstals. Rom Rep Phys 60(4):947–955
Sangawar VS, Bhagat RN (2013) Synthesis and structural properties of poly ethylene oxide complexed with cadmium sulphide. Int J Innov Res Sci Eng Technol 2(11):6539–6547
Palayangoda SS, Nguyen QP (2012) An ATR-FTIR procedure for quantitative analysis of mineral constituent and kerogen in oil shale. Oil Shale 29(4):344–356
Adamczyk NM, Dameron AA, George SM (2008) Molecular layer deposition of Poly (p-phenylene terephthalamide) films using terephthaloyl chloride and p-phenylenediamine. Langmuir 24(5):2081–2089
Shao L, Shu J, Ma R, Shui M, Hou L, Wu KQ, Wang D, Ren Y (2013) Electrochemical characteristics and intercalation mechanism of manganese carbonate as anode material for Lithium-ion batteries. Int J Electrochem Sci 8:1170–1180
Rangelova N, Radev L, Nenkova S, Miranda IM, Salvado MH, Fernandes V, Herzog M (2011) Methylcellulose/SiO2 hybrids: sol-gel preparation and characterization by XRD, FTIR and AFM. Cent Eur J Chem 9(1):112–118
de Carvalho LMG, de Abreu WC, Silva MDGDO, Oliveira JRDO, Oliveira JED, Matos JMED, Moura CVRD, Moura EMD (2013) Heterogeneous catalysis afford biodiesel of babassu, castor oil and blends. J Braz Chem Soc 24(4):550–557
Dhananjay N, Nagabhusana H, Nagabhushana BM, Rudraswamy B, Shivkumara C, Chakradhar RPS (2012) Spherical and rod-like Gd2O3:Eu3+ nanophosphors structural and luminescent properties. Bull Mater Sci 35(4):519–527
Raju GSR, Pavitra E, Yu JS (2014) Pechini synthesis of lanthanide (Eu3+/Tb3+or Dy3+) ions activated BaGd2O4 nanostructured phosphors: an approach for tunable emissions. Phys Chem Chem Phys 16:18124–18140
Carnall WT, Fields PR, Rajnak K (1968) Electronic energy levels in the trivalent lanthanide aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J Chem Phys 49(10):4424–4442
Carnall WT, Fields PR, Rajnak K (1968) Spectral intensities of the trivalent lanthanides and actinides in solution. II. Pm3+, Sm3+, Eu3+, Gd3+, Tb3+, Dy3+and Ho3+. J Chem Phys 49(10):4412–4423
Zhang J, Wang Y, Guo L, Huang Y (2012) Vacuum ultraviolet–ultraviolet, x-ray, and near-infrared excited luminescence properties of SrR2O4:RE3+ (R = Y and Gd; RE = Tb, Eu, Yb, Tm, Er, and Ho). J Am Ceram Soc 95(1):243–249
Wang Z, Zhong J, Liang H, Wang J (2013) Luminescence properties of lutetium based red emitting phosphor NaLu(WO4)2:Eu3+. Opt Mater Express 3(3):418–425
Bettentrup H, Eskola KO, Hölsä J, Kotlov A, Lastusaari M, Malkamäki M (2010) Luminescence properties of Eu3+ and TiIV/ZrIV doped yttrium oxysulfides (Y2O2S:Eu3+, TiIV/ZrIV). IOP Conf Ser 15:012085–012092
Shilpa CJ, Jayaram AK, Dhananjaya N, Nagabhushana H, Prashantha SC, Sunitha DV, Sharma SC, Shivakumara C, Nagabhushana BM (2014) GdAlO3:Eu3+:Bi3+ nanophosphor: Synthesis and enhancement of red emission for WLEDs. Spectrochim Acta A 133:550–558
Musbah SS, Radojević VJ, Borna NV, Stojanović DB, Dramićanin MD, Marinkovć AD, Aleksić RR (2011) PMMA–Y2O3 (Eu3+) nanocomposites: optical and mechanical properties. J Serb Chem Soc 76(8):1153–1161
Zhang Y, Geng D, Shang M, Zhang X, Li X, Cheng Z, Liana H, Lin J (2013) Soft-chemical synthesis and tunable luminescence of Tb3+, Tm3+/Dy3+-doped SrY2O4 phosphors for field emission displays. Dalton Trans 42:4799–4808
Shinde KN, Singh R, Dhoble SJ (2014) Luminescence optimization of Y0.94−x Eu0.06VO4: M x (M = Zn, Al, Bi) red phosphors by the solution combustion method. J Lumin 145:588–594
Chang Y-S, Huang F-M, Tsai Y-Y, Teoh L-G (2009) Synthesis and photoluminescent properties of YVO4:Eu3+ nano-crystal phosphor prepared by pechini process. J Lumin 129:1181–1185
Yang S-H, Yen C-H, Lin C-M, Chiang P-J (2015) Energy transfer mechanism and luminescence properties of color tunable LaPO4:Tm, Eu phosphor. Ceram Int 41:8211–8215
http://www.hunterlab.com/appnotes/an0505.pdf for specific color index. Accessed 20 Mar 2014
http://www.madebydelta.com/imported/images/documents/ICAM/I103%20Dominant%20Wavelength.pdf for the dominant wavelength and color purity of the emitted color. Accessed 12 Mar 2014
Luo W, Liao J, Li R, Chen X (2010) Determination of Judd-Ofelt intensity parameters from the excitation spectra for rare-earth doped luminescent materials. Phys Chem Chem Phys 12:3276–3282
Kodaira CA, Brito HF, Felinto MCF (2003) Luminescence investigation of Eu3+ ion in the RE2(WO4)3 matrix (RE = La and Gd) produced using the pechini method. J Solid State Chem 171:401–407
Som S, Choubey A, Sharma SK (2012) Luminescence studies of rare earth doped yttrium gadolinium mixed oxide phosphor. Phys B 407:3515–3519
Judd BR (1962) Optical absorption intensities of rare-earth ions. Phys Rev 127:750–761
Ofelt GS (1962) Intensities of crystal spectra of rare earth ions. J Chem Phys 37:511–520
Swapna K, Mahamuda S, Sasikala AS, Packiyaraj T, Moorthy LR, Prakash GV (2014) Luminescence characterization of Eu3+ doped Zinc Alumino Bismuth Borate glasses for visible red emission applications. J Lumin 156:80–86
Marcantonatos MD (1986) Multiphonon non-radiative relaxation rates and Judd–Ofelt parameters of lanthanide ions in various solid hosts. J Chem Soc, Faraday Trans 82(2):381–393
Karthikeyan B, Balachandrakumar K, Sakthiraj K, Rajamanickam N (2014–2015) Optical and electrical behavior of nanocrystalline forsterite Mg2−x Zn x SiO4. Int J Chem Tech Res 7(3):1445–1451
Pal PP, Manam J (2014) Enhanced luminescence of ZnO: RE3+ (RE = Eu, Tb) nanorods by Li+ doping and calculations of kinetic parameters. J Lumin 145:340–350
Singh J, Baitha PK, Manam J (2015) Influence of heat treatment on the structural and optical properties of SrGd2O4:Eu3+ phosphor. J Rare Earths 33(10):1040–1050
Pal PP, Manam J (2013) Structural and photoluminescence studies of Eu3+ doped zinc oxide nanorods prepared by precipitation method. J Rare Earths 31(1):37–42
Acknowledgements
The authors acknowledge the financial support from ISM research scholars, funding from Government of India. The authors are grateful to Dr. S. K. Sharma, Department of Applied Physics, ISM Dhanbad for PL and Diffuse Reflectance studies. The authors are also thankful to Prof. A. Subrahmanyam and Deepak Kumar, Department of Physics, IIT Madras for their kind support. The support from Dr. Subrata Das, Department of Chemical Engineering, National Taiwan University, Taipei, Taiwan is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, J., Manam, J. Structural and spectroscopic behaviour of Eu3+-doped SrGd2O4 modified by thermal treatments. J Mater Sci 51, 2886–2901 (2016). https://doi.org/10.1007/s10853-015-9597-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9597-5