Abstract
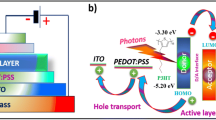
A charm-bracelet-type poly(N -vinylcarbazole) (PVK)–fullerene (C60) donor–acceptor double-cable polymer was synthesized. The covalent attachment of C60 to PVK was confirmed by 1H NMR and FT-IR spectra. Elemental analysis results provided the information that one C60 molecule was present for every seven N-vinylcarbazole units of the polymer corresponding to a fullerene content of 34.76 wt%. The UV–Visible absorption and cyclic voltammetry data indicated that PVK–C60 retained the electronic properties of both PVK and C60. Density functional theory calculations were carried out to determine the LUMO and HOMO energy levels of PVK–C60. The occurrence of photoinduced electron transfer from PVK to C60 was confirmed by photoluminescence techniques. The positioning of the energy levels of PVK–C60 suggests that there is a great scope for achieving a highly efficient molecular heterojunction solar cell with a high open-circuit voltage.












Similar content being viewed by others
References
Chen G, Seo J, Yang C, Prasad PN (2013) Review: nanochemistry and nanomaterials for photovoltaics. Chem Soc Rev 42:8304–8338
Zhang Q, Uchaker E, Candelaria SL, Cao G (2013) Review: nanomaterials for energy conversion and storage. Chem Soc Rev 42:3127–3171
Chen JT, Hsu CS (2011) Review: conjugated polymer nanostructures for organic solar cell applications. Polym Chem 2:2707–2722
Brabec CJ, Sariciftci NS, Hummelen JC (2001) Review: plastic solar cells. Adv Funct Mater 11:15–26
Ruoff RS, Kadish KM, Boulas P, Chen ECM (1995) The relationship between the electron affinities and half-wave reduction potentials of fullerenes, aromatic hydrocarbons and metal complexes. J Phys Chem 99:8843–8850
Yusoff ARBM, Lee SJ, Kim HP, Shneider FK, Silva WJD, Jang J (2014) 8.91% Power conversion efficiency for polymer tandem solar cells. Adv Funct Mater 24:2240–2247
Roncali J (2005) Review: linear π-conjugated systems derivatized with C60-fullerene as molecular heterojunctions for organic photovoltaics. Chem Soc Rev 34:483–495
Cravino A, Sariciftci NS (2003) Organic electronics: molecules as bipolar conductors. Nat Mater 2:360–361
Mozer AJ, Sariciftci NS (2006) Review: conjugated polymer photovoltaic devices and materials. C R Chimie 9:568–577
Cravino A (2007) Review: conjugated polymers with tethered electron-accepting moieties as ambipolar materials for photovoltaics. Poly Int 56:943–956
Miyanishi S, Zhang Y, Hashimoto K, Tajima K (2012) Controlled synthesis of fullerene-attached poly(3-alkylthiophene)-based copolymers for rational morphological design in polymer photovoltaic devices. Macromolecules 45:6424–6437
Lanzi M, Paganin L, Errani F (2012) Synthesis, characterization and photovoltaic properties of a new thiophene-based double-cable polymer with pendent fullerene group. Polymer 53:2134–2145
Tan Z, Hou J, He Y, Zhou E, Yang C, Li Y (2007) Synthesis and photovoltaic properties of a donor-acceptor double-cable polythiophene with high content of C60 pendant. Macromolecules 40:1868–1873
He Y, Hou J, Tan Z, Li Y (2010) Synthesis and photovoltaic properties of polythiophene derivatives with side chains containing C60 end group. J Appl Polym Sci 115:532–539
Thomas RN (1994) Acid catalysed fullerenation of carbazole polymer. J Polym Sci Part A 32:2727–2737
Chen Y, Huang ZE, Cai RF (1996) The synthesis and characterization of C60 chemically modified poly(N-vinylcarbazole). J Polym Sci Part B 34:631–640
Gholamkhass B, Peckham TJ, Holdcroft S (2010) Poly(3-hexylthiophene) bearing pendant fullerenes: aggregation versus self-organization. Polym Chem 1:708–719
Zhang F, Svensson M, Andersson MR, Maggini M, Bucella S, Menna E, Inganä O (2001) Soluble polythiophenes with pendant fullerene groups as double cable materials for photodiodes. Adv Mater 13:1871–1874
Chen M, Li M, Wang H, Qu S, Zhao X, Xie L, Yang S (2013) Side-chain substitution of poly(3-hexylthiophene) (P3HT) by PCBM via postpolymerization: an intramolecular hybrid of donor and acceptor. Polym Chem. 4:550–557
Czichy M, Wagner P, Grzadziel L, Krzywiecki M, Szwajca A, Lapkowski M, Zak J, Officer DL (2014) Electrochemical and photoelectronic studies on C60-pyrrolidine-functionalised poly(terthiophene). Electrochim Acta 141:51–58
Li M, Xu P, Yang J, Yang S (2010) Donor-π-acceptor double-cable polythiophenes bearing fullerene pendant with tunable donor/acceptor ratio: a facile postpolymerization. J Mater Chem 20:3953–3960
Piotrowski P, Zarebska K, Skompska M, Kaim A (2014) Electrodeposition and properties of donor-acceptor double-cable polythiophene with high content of pendant fulleropyrrolidine. Electrochim Acta 148:145–152
Lav TX, Tran-Van F, Aubert PH, Chevrot C (2012) Elaboration and characterization of donor-acceptor polymer through electropolymerization of fullerene substituted N-alkylcarbazole. Synth Met 162:1923–1929
Berton N, Fabre-Francke I, Bourrat D, Chandezon F, Sadki S (2009) Poly(bisthiophene-carbazole-fullerene) double-cable polymer as new donor-acceptor material: preparation and electrochemical and spectroscopic characterization. J Phys Chem B 113:14087–14093
Grazulevicius JV, Strohriegl P, Pielichowski J, Pielichowsk K (2003) Review: carbazole-containing polymers: synthesis, properties and applications. Prog Polym Sci 28:1297–1353
Davidge H (1959) Poly-N-vinylcarbazole. II. Preparation, moulding and dielectric properties. J Appl Chem 9:553–560
Chapiro A, Hardy G (1962) Radiochemical polymerization of N-vinylcarbazole in liquid and solid phase. J Chim Phys Phys Chim Biol 59:993–998
Tazuke S (1973) Initiation of photopolymerization by charge transfer interactions. Pure Appl Chem 34:329–352
Atmaca L, Kayihan I, Yagci Y (2000) Photochemically and thermally induced radical promoted cationic polymerization using allyl phosphonium salts. Polymer 41:6035–6041
Ohmori Y, Kajii H, Sawatani T, Ueta H, Yoshino K (2001) Enhancement of electroluminescence utilizing confined energy transfer for red light emission. Thin Solid Films 393:407–411
Wang G, Yuan C, Yu H, Wei Y (1995) Importance of poly(N-vinylcarbazole) dopant to poly(3-octylthiophene) electroluminescence. J Appl Phys 78:2679–2683
Hu B, Yang Z, Karasz FE (1994) Electroluminescence of pure poly(N-vinylcarbazole) and its blends with a multiblock copolymer. J Appl Phys 76:2419–2422
Zhang C, Seggern HV, Pakbaz K, Kraabel P, Schmidt HW, Heeger AJ (1994) Blue electroluminescent diodes utilizing blends of poly(p-phenylenevinylene) in poly(9-vinylcarbazole). Synth Met 62:35–40
Liang C, Li W, Hong Z, Liu X, Peng J, Liu L, Lu Z, Xie M, Liu Z, Wu J, Zhao D (1997) Energy transfer process from polymer to rare earth complexes. Synth Met 91:151–154
Zheng H, Zhang R, Wu F, Tian W, Chen J (1999) Photoluminescence and electroluminescence properties of coumarin-uria/poly(N-vinylcarbazole) blends. Synth Met 100:291–295
Wang G, Qian S, Xu J, Wang W, Liu X, Lu X, Li F (2000) Enhanced photovoltaic response of PVK/C60 composite films. Phys B 279:116–119
Wang Y, Herron N (1992) Photoconductivity of CdS nanocluster-doped polymers. Chem Phys Lett 200:71–75
West DP, Rahn MD, Im C, Bassler H (2000) Hole transport through chromophores in a photorefractive polymer composite based on poly(N-vinylcarbazole). Chem Phys Lett 326:407–412
Zhang Y, Spencer CA, Ghosal S, Casstevens MK, Burzynski R (1994) Thiapyrylium dye sensitization of photorefractivity in a polymer composite. Appl Phys Lett 64:1908–1910
Gunter P, Huingnard JP (1998) Photorefractive materials and their applications. Springer, Berlin
Ling QD, Lim SL, Song Y, Zhu CX, Chan DSH, Kang ET, Neoh KG (2007) Nonvolatile polymer memory devices based on bistable electrical switching in a thin film of poly(N-vinylcarbazole) with covalently bonded C60. Langmuir 23:312–319
Shirota Y, Kakuta T, Kanega H, Mikawa H (1985) Rectification and photovoltaic properties of a schottky barrier cell using electrochemically-doped poly(N-vinylcarbazole). J Chem Soc Chem Commun 17:1201–1202
Kim HS, Kim CH, Ha CS, Lee JK (2001) Organic solar cell devices based on PVK/porphyrin system. Synth Met 117:289–291
Ikeda N, Miyasaka T (2005) A solid-state dye-sensitized photovoltaic cell with a poly(N-vinylcarbazole) hole transporter mediated by an alkali iodide. Chem Commun 14:1886–1888
Kaune G, Wang W, Metwalli E, Ruderer M, Robner R, Roth SV, Muller-Buschbaum P (2008) Layered TiO2: PVK nanocomposites thin films for photovoltaic applications. Eur Phys J E 26:73–79
Davenas J, Barlier V, Legare VB, Canut B, Rybak A, Slazak A, Jung J (2010) Hybrid polymer/TiO2 films by in situ hydrolysis condensation of titanium alkoxide precursors for photovoltaic transparent windows. Phys Status Solidi A 207:1627–1630
Tria MC, Liao KS, Alley N, Curran S, Advincula R (2011) Electrochemically crosslinked surface-grafted PVK polymer brushes as a hole transport layer for organic photovoltaics. J Mater Chem 21:10261–10264
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA (2009) Gaussian 09, revision C.01. Gaussian, Inc, Wallingford, CT
Olah GA, Bucsi I, Lambert C, Aniszfeld R, Trivedi NJ, Sensharma DK, Prakash GKS (1991) Polyarenefullerenes, C60(H–Ar)n, obtained by acid-catalyzed fullerenation of aromatics. J Am Chem Soc 113:9387–9388
Watanabe A, Kawato T, Matsuda M, Ito O (1997) Formation of semiconducting polymer film by laser CVD. J Photopolym Sci Technol 10:197–204
Penwell RC, Prest WM Jr (1978) Orientation in poly(N-vinylcarbazole) by melt extrusion. Polymer 19:537–541
Ballav N (2005) Some experimental results on poly(N-vinylcarbazole)-buckminsterfullerene (C60) nanocomposites system. Mater Lett 59:3419–3422
Ji HX, Hu JS, Wan LJ, Tang QX, Hu WP (2008) Controllable crystalline structure of fullerene nanorods and transport properties of an individual nanorod. J Mater Chem 18:328–332
Leach S, Vervloet M, Despres A, Breheret E, Hare JP, Dennis TJ, Kroto HW, Taylor R, Walton DRM (1992) Electronic spectra and transitions of the fullerene C60. Chem Phys 160:451–466
Hirsch A, Soi A, Karfunhel HR (1992) Titration of C60: a method for the synthesis of organofullerenes. Angew Chem Int Ed 31:766–768
Nowacki B, Iamazaki E, Cirpan A, Karasz F, Atvars TDZ, Akcelrud L (2009) Highly efficient polymer blends from a polyfluorene derivative and PVK for LEDs. Polymer 50:6057–6064
Beheshtian J, Peyghan AA, Bagheri Z (2012) Theoretical investigation of C60 fullerene functionalization with tetrazine. Comput Theor Chem 992:164–167
Wang TL, Yang CH, Shieh YT, Yeh AC (2009) Synthesis of CdSe-Poly(N-vinylcarbazole) nanocomposites by atom transfer radical polymerization for potential optoelectronic applications. Macromol Rapid Commun 30:1679–1683
Acknowledgements
The authors gratefully acknowledge the financial assistance from the Department of Science and Technology Govt. of India, New Delhi for this research project (SR/S2/CMP-58/06). They are grateful to Dr. V. Hakkim, Central Leather Research Institute, Chennai for helping with the DFT calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramar, A., Saraswathi, R. Synthesis and characterization of a charm-bracelet-type poly(N-vinylcarbazole)–C60 double-cable polymer. J Mater Sci 50, 3740–3749 (2015). https://doi.org/10.1007/s10853-015-8936-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8936-x