Abstract
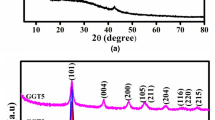
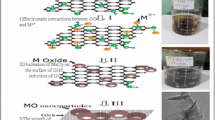
Strong increase in electrical conductivity of graphene oxide (GO) (I ≈ 10−9 A) is found by addition of Ni nanoparticles (NiNPs) preliminarily solved by HCl (Nisol) (I ≈ 10−4 A) or powder (Nipow) obtained from this solution (I ≈ 10−6 A), while simply mixing GO with NiNPs an insulator similar to pure GO is obtained. Thus, Nisol and Nipow can be used to transform GO from insulator to semiconductor. One of the transformation mechanisms is Ni as spillover. At the same time, different kinds of the magnetic response are obtained on GO and reduced GO (rGO) samples with and without Ni. Weak paramagnetic response is detected in pure GO. Stronger paramagnetic behavior is observed for GO and rGO mixed with Nisol or Nipow. Pure rGO sample shows weak ferromagnetism represented by slim but visible hysteresis with remnant magnetization M r of 0.05 emu/g. GO with NiNPs presents clear hysteresis with M r of 2.8 emu/g, while sample prepared by addition of NiNPs to rGO presents the largest hysteresis with M r as high as 11.8 emu/g. Thus, the optimal procedure to obtain the magnetic response requested for particular application can be chosen.









Similar content being viewed by others
References
Britnell L, Gorbachev RV, Geim AK, Ponomarenko LA, Mishchenko A, Greenaway MT, Fromhold TM, Novoselov KS, Eaves L (2013) Resonant tunnelling and negative differential conductance in graphene transistors. Nat Commun 4:1794-1–1794-5
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Yamashiro Y, Ohno Y, Maehashi K, Inoue K, Matsumoto K (2012) Electric-field-induced band gap of bilayer graphene in ionic liquid. J Vac Sci Technol B 30:03D111-1–03D111-5
Craciun MF, Russo S, Yamamoto M, Oostinga JB, Morpurgo AF, Tarucha S (2009) Trilayer graphene is a semimetal with a gate-tunable band overlap. Nat Nanotechnol 4:383–388
Eda G, Chhowalla M (2010) Chemically derived graphene oxide: towards large-area thin film electronics and opto-electronics. Adv Mater 22:2392–2415
Han MY, Ozyilmaz B, Zhang YB, Kim P (2007) Energy band-gap engineering of graphene nanoribbons. Phys Rev Lett 98:206805-1–206805-4
Wang XR, Ouyang YJ, Li XL, Wang HL, Guo J, Dai HJ (2008) Room-temperature all-semiconducting sub-10-nm graphene nanoribbon field-effect transistors. Phys Rev Lett 100:206803-1–206803-4
Han KH, Spemann D, Esquinazi P, Höhne R, Riede V, Butz T (2003) Ferromagnetic spots in graphite produced by proton irradiation. Adv Mater 15:1719–1722
Vozmediano MAH, Lopez-Sancho MP, Stauber T, Guinea F (2005) Local defects and ferromagnetism in graphene layers. Phys Rev B 72:155121-1–155121-5
Fujita M, Wakabayashi K, Nakada K, Kusakabe K (1996) Peculiar localized state at zigzag graphite edge. J Phys Soc Jpn 65:1920–1923
Shibayama Y, Sato H, Enoki T, Endo M (2000) Disordered magnetism at the metal-insulator threshold in nano-graphite-based carbon materials. Phys Rev Lett 84:1744–1747
Park N, Yoon M, Berber S, Ihm J, Osawa E, Tománek D (2003) Magnetism in all-carbon nanostructures with negative gaussian curvature. Phys Rev Lett 91:237204-1–237204-4
Pisani L, Montanari B, Harrison NM (2008) Predicted to be a room temperature ferromagnetic semiconductor. New J Phys 10:033002-1–033002-10
Son YW, Cohen ML, Louie SG (2006) Half-metallic graphene nanoribbons. Nature 444:347–349
Hong J, Bekyarova E, de Heer WA, Haddon RC, Khirzoev S (2013) Chemically engineered graphene-based 2D organic molecular magnet. ACS Nano 7:10011–10022
Okhay O, Krishna R, Salimian M, Titus E, Gracio J, Guerra LM, Ventura J (2013) Conductivity enhancement and resistance changes in polymer films filled with reduced graphene oxide. J Appl Phys 113:064307-1–064307-5
Mei X, Ouyang J (2011) Ultrasonical-assisted ultrafast reduction of graphene oxide by zinc powder at room temperature. Carbon 49:5389–5397
Wang Z, Hu Y, Yang W, Zhou M, Hu X (2012) Facile one-step microwave-assisted route towards Ni nanospheres/reduced graphene oxide hybrids for non-enzymatic glucose sensing. Sensor 12:4860–4869
Moon IK, Lee J, Ruoff RS, Lee H (2010) Reduced graphene oxide by chemical graphitization. Nat Commun 1:73–79
Choi EY, Han TH, Hong J, Kim JE, Lee SH, Kim HW, Kim SO (2010) Noncovalent functionalization of graphene with end-functional polymers. Mater Chem 20:1907–1912
Khenfouch M, Baïtoul M, Aarab H, Maaza M (2012) Vibrational and thermal properties of confined graphene nanosheets in an individual polymeric nanochannel by electrospinning. Graphene 1:15–20
Zheng L, Li Z, Bourdo S, Watanabe F, Ryerson CC, Biris AS (2011) Catalytic hydrogentation of graphene films. Chem Commun 47:1213–1215
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224
Fujimori A, Tokura Y (1995) Spectroscopy of mott insulators and correlated metals. Springer, Berlin
Lee JD (2008) Concise inorganic chemistry, 5th edn. Oxford University Press, London
Housecroft CE, Sharpe AG (2008) Inorganic chemistry. Pearson Prentice Hall, Upper Saddle River
Krishna R, Titus E, Costa LC, Menezes JCJMDS, Correia MRP, Pinto S, Ventura J, Araújo JP, Cavaleiro JAC, Gracio JJA (2012) Facile synthesis of hydrogenated reduced graphene oxide via hydrogen spillover mechanism. J Mater Chem 22:10457–10459
Goethel PJ, Yang RT (1987) Mechanism of catalyzed graphite oxidation by monolayer channeling and monolayer edge recession. J Catal 108:156–158
Mittendorfer F, Hafner J (2002) Hydrogenation of benzene on Ni(111)—a DFT study. J Phys Chem B 106:13299–13305
Solomons TW, Fryhle CB (2004) Organic chemistry, 8th edn. Wiley, New York
Krishna R, Titus E, Salimian M, Okhay O, Rajendran S, Rajkumar A, Sousa JMG, Ferreira ALC, Gil GC, Gracio J (2012) Hydrogen storage for energy application. In: Liu J (ed) Hydrogen storage. Winchester, Intech Open, pp 243–266
Jeong DS, Thomas R, Katiyar RS, Scott JF, Kohlstedt H, Petraru A, Hwang CS (2012) Emerging memories: resistive switching mechanisms and current status. Rep Prog Phys 75:076502-1–076502-31
Park G-S, Li X-S, Kim D-C, Jung R-J, Lee M-J, Seo S (2007) Observation of electric-field induced Ni filament channels in polycrystalline NiOx film. Appl Phys Lett 91:222103-1–222103-3
Morisaki H, Saigo K, Shintani S, Yazawa K (1974) Memory-switching in amorphous carbon films. J Non Cryst Solids 15:531–534
Fu D, Xie D, Zhang CH, Zhang D, Niu JB, Qian H, Liu LT (2010) Preparation and characteristics of nanoscale diamond-like carbon films for resistive memory applications. Chin Phys Lett 27:098102-1–098102-4
Wang Y, Huang Y, Song Y, Zhang X, Ma Y, Liang J, Chen Y (2009) Room-temperature ferromagnetism of graphene. Nano Lett 9:220–224
Ramakrishna Matte HSS, Subrahmanyam KS, Rao CNR (2009) Presence of both ferromagnetic and antiferromagnetic features and other aspects. J Phys Chem C Lett 113:9982–9985
Kimishima Y, Miyata N, Akutsu N, Ichiyanagi Y, Hagiwara M (1992) Magnetic study on the precipitate from the aqueous solutions of NiCl2·6H2O and Na2SiO3·nH2O. J Magn Magn Mater 104–107:781–782
Acknowledgements
Olena Okhay acknowledges FCT for financial support (SFRH/BD/77704/2011). This work was funded also by the EU’s 7th Framework program IFOX (NMP3-LA-2010 246102), the Graduate School of Excellence MAINZ (GSC 266 Mainz), the German Science Foundation (DFG SPP 1459 Graphene), and the ERC (2007-Stg 208162). Alexander Tkach acknowledges also funds by FEDER through Programa Operacional Factores de Competitividade—COMPETE and national funds through FCT within CICECO Project—FCOMP-01-0124-FEDER-037271 (FCT Ref. PEst-C/CTM/LA0011/2013) and independent researcher grant IF/00602/2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okhay, O., Krishna, R., Tkach, A. et al. Drastic modification of graphene oxide properties by incorporation of nickel: a simple inorganic chemistry approach. J Mater Sci 50, 3425–3433 (2015). https://doi.org/10.1007/s10853-015-8901-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8901-8