Abstract
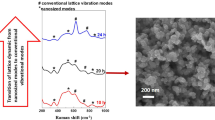
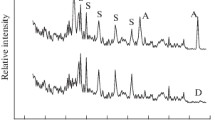
An efficient method was developed for fabricating a highly porous nanoforest structure composed of ZnO/C core–shell hexagonal nanosheets (HNSs). Compact thermolysis of zinc acetate dihydrate in a sealed bath reactor at 400 °C over 20 h yielded the nanoforest structures. A carbon shell layer coating was applied in situ during the growth of the ZnO nanosheet core. The structures, morphologies, growth processes, compositions, and binding characteristics of the ZnO/C core–shell HNS nanoforests were analyzed using multi-purpose high-performance X-ray diffraction (XRD), scanning electron microscopy, energy-dispersive X-ray spectroscopy, Raman spectroscopy, transmission electron microscopy, and X-ray photoelectron spectroscopy (XPS) techniques. XRD and XPS results suggest the existence of oxygen vacancy defects in the core surface of ZnO/C core–shell. The ZnO/C core–shell HNS nanoforests exhibited strong absorption features from the visible to the near-IR region (400–1670 nm), and the nanoforest films showed high electrical conductivity.












Similar content being viewed by others
References
Xai Y, Yang P, Sun Y, Wu Y, Mayers B, Gates B, Yin Y, Kim F, Yan H (2003) One-dimensional nanostructures: synthesis, characterization, and applications. Adv Mater 15:353–389
Xei X, Li Y, Liu ZQ, Haruta M, Shen W (2009) Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458:746–749
Qian HS, Yu SH, Luo LB, Gong JY, Fei LF, Liu XM (2006) Synthesis of uniform te@carbon-rich composite nanocables with photoluminescence properties and carbonaceous nanofibers by the hydrothermal carbonization of glucose. Chem Mater 18:2102–2108
Caruso F (2001) Nanoengineering of particle surfaces. Adv Mater 13:11–22
Jiang L, Gao L (2005) Fabrication and characterization of ZnO-coated multi-walled carbon nanotubes with enhanced photocatalytic activity. Mater Chem Phys 91:313–316
Kalpana D, Omkumar KS, Kumar SS, Renganathan NG (2006) A novel high power symmetric ZnO/carbon aerogel composite electrode for electrochemical supercapacitor. Electrochim Acta 52:1309–1315
Greene LE, Law M, Tan DH, Montano M, Goldberger J, Somorjai G, Yang P (2005) General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett 5:1231–1236
Zhang Y, Wang L, Liu X, Yan Y, Chen Ch, Zhu J (2005) Synthesis of nano/micro zinc oxide rods and arrays by thermal evaporation approach on cylindrical shape substrate. J Phys Chem B 109:13091–13093
Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291:1947–1949
Pan Z, Budai JD, Dai ZR, Liu W, Paranthaman MP, Sheng Dai S (2009) Zinc oxide microtowers by vapor phase homo epitaxial regrowth. Adv Mater 21:890–896
Martin PM, Good MS, Johnston JW, Bond LJ, Crawford SL (2000) Piezoelectric films for 100-MHz ultrasonic transducers. Thin Solid Films 379:253–258
Xu JQ, Pan QY, Shun YA, Tian ZZ (2000) Grain size control and gas sensing properties of ZnO gas sensor. Sens Actuators B 66:277–279
Huang MH, Mao S, Feick H, Yan H, Wu YY, Kind H, Weber E, Russo R, Yang PD (2001) Room-temperature ultraviolet nanowire nanolasers. Science 292:1897–1899
Dai Y, Zhang Y, Li QK, Nan CW (2002) Synthesis and optical properties of tetrapod-like zinc oxide nanorods. Chem Phys Lett 358:83–86
Li WJ, Shi EW, Zhong WZ, Yin ZW (1999) Growth mechanism and growth habit of oxide crystals. J Cryst Growth 203:186–196
Chen Y, Bagnall D, Yao T (2000) ZnO as a novel photonic material for the UV region. Mater Sci Eng B 75:190–198
Saito N, Haneda H, Sekiguchi T, Ohashi N, Sakaguchi I, Koumoto K (2002) Low-temperature fabrication of light-emitting zinc oxide micropatterns using self-assembled monolayers. Adv Mater 14:418–421
Liang S, Sheng H, Liu Y, Hio Z, Lu Y, Shen H (2001) ZnO Schottky ultraviolet photodetectors. J Cryst Growth 225:110–113
Lin Y, Zhang Z, Tang Z, Yuan F, Li J (1999) Characterization of ZnO-based varistors prepared from nanometer precursor powders. Adv Mater 9:205–209
Zhang H, Yang D, Ma X, Yujie Ji, Xu J, Que D (2004) Synthesis of flower-like ZnO nanostructures by an organic-free hydrothermal process. Nanotechnology 15:622–626
Liu J, Li Y, Ding R, Jiang j, Hu Y, Ji X, Chi Q, Zhu Z, Huang X (2009) Carbon/ZnO nanorod array electrode with significantly improved lithium storage capability. J Phys Chem C 113:5336–5339
Ding R, Liu J, Jiang J, Li Y, Hu Y, Ji X, Chi Q, Wu F, Huang X (2009) High surface area ZnO–carbon composite tubular arrays based on the Kirkendall effect and in situ Zn evaporation. Chem Commun 30:4548–4550
Hossain MM, Mamun AHA, Hahn JR (2012) Fabrication of solid cylindrical-shaped microtowers of ZnO/C core–shell hexagonal nanorods by thermolysis. J Phys Chem C 116:23153–23159
Paraguay F, Estrada W, Acosta DR, Andrade E, Miki-Yoshida M (1999) Growth, structure and optical characterization of high quality ZnO thin films obtained by spray pyrolysis. Thin Solid Films 350:192–202
Pol VG, Calderon-Moreno JM, Thiyagarajan P (2008) Facile synthesis of novel photoluminescent ZnO micro- and nanopencils. Langmuir 24:13640–13645
Watcharotone S, Dikin DA, Stankovich S, Piner R, Jung I, Dommett GHB, Evmenenko G, Wu SE, Chen SF, Liu CP, Nguyen ST, Ruoff RS (2007) Graphene–silica composite thin films as transparent conductors. Nano Lett 7:1888–1892
Wang Z-M, Wang WD, Coombs N, Soheilnia N, Ozin GA (2010) Graphene oxide–periodic mesoporous silica sandwich nanocomposites with vertically oriented channels. ACS Nano 4:7437–7450
Wang HL, Cui LF, Yang Y, Casalongue HS, Robinson JT, Liang YY, Cui Y, Dai HJ (2010) Mn3O4-graphene hybrid as a high-capacity anode material for lithium ion batteries. J Am Chem Soc 132:13978–13980
Son DI, Kwon BW, Park DH, Seo WS, Yi Y, Angadi B, Lee CL, Choi WK (2012) Emissive ZnO–graphene quantum dots for white-light-emitting diodes. Nat Nanotechnol 7:465–471
Bairamov BH, Heinrich A, Irmer G, Toporov VV, Ziegler E (1983) Raman study of phonon halfwidths and the phonon–plasmon in ZnO. Phys Status Solidi B 119:227–234
Lv Y, Yu L, Huang H, Feng Y, Chen D, Xie X (2012) Application of the soluble salt-assisted route to scalable synthesis of ZnO nanopowder with repeated photocatalytic activity. Nanotechnology 23:065402
Salavati-Niasari M, Davar F, Bazarganipour M (2010) Synthesis, characterization and catalytic oxidation of para-xylene by manganese (111) Schiff base complex on functionalized multi-wall carbon nanotubes. Dalton Trans 39:7330–7337
Teng CC, Ma CCM, Lu CH, Yang SY, Lee SH, Hsiao MC, Yen MY, Chiou KC, Lee TM (2011) Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 49:5107–5116
Wang J, Wang Z, Huang B, Ma Y, Liu Y, Qin X, Zhang X, Ying Dai Y (2012) Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl Mater Interfaces 4:4024–4030
Yin X, Hesselink L, Liu Z et al (2004) Large positive and negative lateral optical beam displacements due to surface plasmon resonance. App Phys Lett 85:372–374
Sherry LJ, Jin R, Mirkin CA et al (2006) Localized surface plasmon resonance spectroscopy of single silver triangular nanoprisms. Nano Lett 6:2060–2065
Baida H, Billaud P, Marhaba S et al (2009) Quantitative determination of the size dependence of surface plasmon resonance damping in single Ag@ SiO2 nanoparticles. Nano Lett 9:3463–3469
Zhang Q, Chou TP, Russo B, Jenekhe SA, Cao G (2008) Polydisperse aggregates of ZnO nanocrystallites: a method for energy-conversion-efficiency enhancement in dye-sensitized solar cells. Adv Funct Mater 18:1654–1660
Zhang Q, Chou TP, Russo B, Jenekhe SA, Cao G (2008) Aggregation of ZnO nanocrystallites for high conversion efficiency in dye-sensitized solar cells. Angew Chem Int Ed 47:2402–2406
Horiuchi H, Katoh R, Hara K, Yanagida M, Murata S, Arakawa H, Tachiya M (2003) Electron injection efficiency from excited N3 into nanocrystalline ZnO films: effect of (N3–Zn2+) aggregate formation. J Phys Chem B 107:2570–2574
Shin YJ, Lee JH, Park JH, Park NG (2007) Enhanced photovoltaic properties of SiO2-treated ZnO nanocrystalline electrode for dye sensitized solar cell. Chem Lett 36:1506–1507
Law M, Greene LE, Radenovic A, Kuykendall T, Liphardt J, Yang PD (2006) ZnO–Al2O3 and ZnO–TiO2 core–shell nanowire dye-sensitized solar cells. J Phys Chem B 110:22652–22663
Acknowledgements
This work was supported by grants from the Korean government (NRF, MSIP, 2010-0024254 and 2007-0056095), and HS and MMH were supported by the BK21 PLUS program. In addition, Dr. B-C. Ku was supported by a grant from KIST institutional program (2Z04250).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hossain, M.M., Shima, H., Ku, BC. et al. Nanoforests composed of ZnO/C core–shell hexagonal nanosheets: fabrication and growth in a sealed thermolysis reactor and optical properties. J Mater Sci 50, 93–103 (2015). https://doi.org/10.1007/s10853-014-8569-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8569-5