Abstract
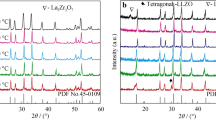
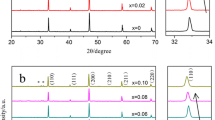
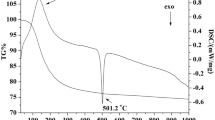
Composites of Strontium- and Magnesium-doped lanthanum gallate, La0.9Sr0.1Ga0.8Mg0.2O2.85 (LSGM), and yttria-stabilized zirconia, Zr0.9Y0.1O1.95 (YSZ), with weight ratios of 9.5:0.5, 9:1 and 8.5:1.5 were first prepared by co-precipitation route. The component compounds, LSGM and YSZ were also prepared by the same route for comparative study. X-ray Rietveld analyses revealed that the sintered LSGM–YSZ composites contain mainly perovskite orthorhombic LSGM phase along with fluorite YSZ phase similar to that of the cubic zirconia. Scanning Electron Microscopic image of the composite depicted the spherical- and oval-shaped grains. XPS spectra of the composite exhibited the LSGM along with a trace amount of the YSZ constituents. Electrical conductivity of the three systems was measured in the frequency range 20 Hz–1 MHz and in the temperature range of 400–700 °C. LSGM–YSZ composite electrolyte with 9:1 ratio was found to exhibit enhanced electrical conductivity in comparison to LSGM and YSZ systems. Moreover, it exhibited lower activation energy than that of their individual components.








Similar content being viewed by others
References
Ishihara T, Matsuda H, Takita Y (1994) Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor. J Am Chem Soc 116:3801–3803
Feng M, Goodenough JB (1994) A superior oxide-ion electrolyte. Eur J Solid State Inorg Chem 31:663–669
Huang P, Petric A (1996) Superior oxygen ion conductivity of lanthanum gallate doped with strontium and magnesium. J Electrochem Soc 143:1644–1648
Cristiani C, Zampori L, Latorrata S, Pelosato R, Dotelli G, Ruffo R (2009) Carbonate coprecipitation synthesis of Sr- and Mg-doped LaGaO3. Mater Lett 63:1892–1894
Chen WX, Nie HW, Huang WH, Zheng R, Tu HY, Wen TL (2003) La0.6Sr0.4Co0.8Mn0.2O3-δ cathode for an intermediate temperature SOFC. J Mater Sci Lett 22:651–653
Shaula AL, Kharton VV, Marques FMB (2004) Phase interaction and oxygen transport in La0.8Sr0.2Fe0.8Co0.2O3–(La0.9Sr0.1)0.98Ga0.8Mg0.2O3 composites. J Eur Ceram Soc 24:2631–2639
Sakai N, Horita T, Yamaji K, Brito ME, Yokokawa H, Kawakami A, Matsuoka S, Watanabe N, Ueno A (2006) Interface stability among solid oxide fuel cell materials with perovskite structures. J Electrochem Soc 153:A621–A625
Liang CC (1973) Conduction characteristics of the lithium iodide-aluminium oxide solid electrolytes. J Electrochem Soc 120:1289–1292
Maier J (1985) Space charge regions in solid two-phase systems and their conduction contribution—I. Conductance enhancement in the system ionic conductor-‘inert’ phase and application on AgC1:Al2O3 and AgC1:SiO2. J Phys Chem Solids 46:309–320
Mishima Y, Mitsuyasu H, Ohtaki M, Eguchi K (1998) Solid oxide fuel cell with composite electrolyte consisting of samaria-doped ceria and yttria-stabilized zirconia. J Electrochem Soc 145:1004–1007
Jang WS, Hyun SH, Kim SG (2002) Preparation of YSZ/YDC and YSZ/GDC composite electrolytes by the tape casting and sol–gel dip-drawing coating method for low-temperature SOFC. J Mater Sci 37:2535–2541. doi:10.1023/A:1015451910081
Wei B, Lü Z, Huang X, Li S, Ai G, Liu Z, Su W (2006) Electrochemical characteristics of Ba0.5Sr0.5Co0.8Fe0.2O3−δ –Sm0.2Ce0.8O1.9 composite materials for low-temperature solid oxide fuel cell cathodes. Mater Lett 60:3642–3646
Hui S, Roller J, Yick S, Zhang X, Deces-Petit C, Xie Y, Maric R, Ghosh D (2007) A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J Power Sources 172:493–502
Zhou XD, Scarfino B, Anderson HU (2004) Electrical conductivity and stability of CGO–YSZ solid solutions. Solid State Ion 175:19–22
Lee JH, Yoon SM, Kim BK, Lee HW, Song HS (2002) Electrical conductivity and defect structure of CeO2–ZrO2 mixed oxide. J Mater Sci 37:1165–1171. doi:10.1023/A:1014363304942
Xu D, Liu X, Wang D, Yi G, Gao Y, Zhang D, Su W (2007) Fabrication and characterization of SDC-LSGM composite electrolytes material in IT-SOFC. J Alloys Compd 429:292–295
Jo SH, Muralidharan P, Kim DK (2010) Electrical conductivity studies on the LSGM–CGO composite electrolytes. J Alloys Compd 491:416–419
Li S, Li Z, Bergman B (2010) Lanthanum gallate and ceria composite as electrolyte for solid oxide fuel cells. J Alloys Compd 492:392–395
Hao G, Liu X, Wang H, Be H, Pei L, Su W (2012) Performance of Ce0.85Sm0.15O1.9–La0.9Sr0.1Ga0.8Mg0.2O2.85 composite electrolytes for intermediate-temperature solid oxide fuel cells. Solid State Ion 225:81–84
Liu YL, Hagen A, Barfod R, Chen M, Wang HJ, Poulsen FW, Hendriksen PV (2009) Microstructural studies on degradation of interface between LSM–YSZ cathode and YSZ electrolyte in SOFCs. Solid State Ion 180:1298–1304
Yang CCT, Wei WCJ, Roosen A (2004) Reaction kinetics and mechanisms between La0.65Sr0.3MnO3 and 8 mol% Yttria-stabilized zirconia. J Am Ceram Soc 87:1110–1116
Drennan J, Zelizk V, Hay D, Ciacchi FT, Rajendran S, Badwal SPS (1997) Characterisation, conductivity and mechanical properties of the oxygen-ion conductor La0.9Sr0.1Ga0.8Mg0.2O3−δ . J Mater Chem 7:79–83
Vasylechko L et al (2003) Crystal structure, thermal expansion and conductivity of anisotropic La1–x Sr x Ga1–2x Mg2x O3–y (x = 0:05; 0.1) single crystals. J Solid State Chem 172:396–441
Shkerin SN, Kuznetsov MV, Kalashnikova NA (2003) X-ray Photoelectron Spectroscopy of the Surface of Solid Electrolyte La0.88Sr0.12Ga0.82Mg0.18O3−α . Russ J Electrochem 39:591–599
Raghvendra, Singh RK, Singh P (2014) Synthesis of La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolyte via ethylene glycol route and its characterizations for IT-SOFC. Ceram Int 40:7177–7184
Jiang Y, Gao J, Liu M, Wang Y, Meng G (2007) Fabrication and characterization of Y2O3 stabilized ZrO2 films deposited with aerosol-assisted MOCVD. Solid State Ion 177:3405
Waser R (1991) Bulk conductivity and defect chemistry of acceptor-doped strontium titanate in the quenched state. J Am Ceram Soc 74:1934–1940
Elliot SR (1987) AC conduction in amorphous-chalcogenide and pnictide semiconductors. Adv Phys 36:135–217
Singh P, Raghvendra, Parkash O, Kumar D (2011) Scaling of low-temperature conductivity spectra of BaSn 1− x Nb x O3 (x ≤ 0.100): temperature and compositional-independent conductivity. Phys Rev B 84:174306–174312
Jonscher AK (1977) The ‘universal’ dielectric response. Nature 267:673–679
Jonscher AK (1983) Dielectric relaxation in solids. Chelsea Dielectric Press, London
Almond DP, West AR (1983) Anomalous conductivity prefactors in fast ion conductors. Nature 306:456–457
Almond DP, Duncan GK, West AR (1983) The determination of hopping rates and carrier concentrations in ionic conductors by a new analysis of ac conductivity. Solid State Ion 8:159–164
Almond DP, West AR (1983) Mobile ion concentrations in solid electrolytes from an analysis of A.C. conductivity. Solid State Ion 9&10:277–282
Sidebottom DL (1999) Ionic conductivity in glasses: is the window effect statistically relevant? J Non Cryst Solids 244:223–231
Singh P, Singh BP, Raghvendra (2012) Dispersion in AC conductivity of fragile glass melts near glass transition temperature. Solid State Ion 227:39–45
Almond DP, West AR (1987) The activation entropy for transport in ionic conductors. Solid State Ion 23:27–35
Maier J (1986) On the conductivity of polycrystalline materials. Ber Bunsenges Phys Chem 90:26–33
Knauth P (2000) Ionic conductor composites: theory and materials. J Electroceram 5:111–125
Jiang S, Wagner JB Jr (1995) A theoretical model for composite electrolytes—I. space charge layer as a cause for charge-carrier enhancement. J Phys Chem Solids 56:1101–1111
Macdonald JR (1987) Impedance spectroscopy: emphasizing solid materials and systems. Wiley Co., New York
Joshi AV, Wagner JRJB (1975) Electrochemical studies on single crystalline CuCl solid electrolyte. J Electrochem Soc 122:1071–1080
Fabbri E, Pergolesi D, Traversa E (2010) Ionic conductivity in oxide heterostructures: the role of interfaces. Sci Technol Adv Mater 11:054503–054512
Tietz F (1999) Thermal expansion of SOFC materials. Ionics 5:129–139
Acknowledgements
We acknowledge DST-SERC for funding this work through its project sanction letter No. SR/FTP/ETA-0005/2010. We are thankful to Prof. ASK Sinha and Prof. O.P. Pandey for providing XPS and TEC measurement facility, respectively. Mr. Raghvendra is grateful to MHRD for providing teaching assistantship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raghvendra, Singh, R.K. & Singh, P. Electrical conductivity of LSGM–YSZ composite materials synthesized via coprecipitation route. J Mater Sci 49, 5571–5578 (2014). https://doi.org/10.1007/s10853-014-8265-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8265-5