Abstract
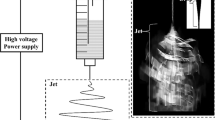
Electro-spraying/-spinning is a facile technique for the generation of nanometer to micrometer scale structures such as thin films, particles, beaded fibers, and fibers that can find a wide spectrum of applications. In this work, poly[(R)-3-hydroxybutyric acid] (PHB), which is a well-known biocompatible and biodegradable polymer, was studied with the objective of understanding the influence of PHB concentration and solvent type on the formation of the aforementioned structures when electro-sprayed/-spun. Solutions of varying concentrations of PHB (1–14 %) in chloroform, dichloroethane, and chloroform:dichloroethane (1:1) were electro-sprayed/-spun to fabricate different types of structures. It was observed that at any specific concentration, solvent properties significantly influenced formation of different kinds of structures with chloroform-based systems leading to more porous structures. Further, an increase in the concentration of PHB in solution while keeping the solvent type constant led to a gradual change in the morphology from thin films (1.5 %) to particles (1.0–2 %) to beaded fibers (3–10 %) and to fibers (14 %).Therefore, it was inferred that a variety of micro-structures of PHB can be fabricated by modulating the polymer solution concentration and solvent system. The results provide an improved understanding of the relationship between solution properties and micro-structures obtained by electro-spraying/-spinning of PHB. In addition, it also provides a method to fabricate micro-structures with diverse morphologies which can have significant implications in biomedical applications such as drug delivery and tissue engineering.









Similar content being viewed by others
References
Salleo A, Street RA (2003) Light-induced bias stress reversal in polyfluorene thin-film transistors. J Appl Phys 94:471–479
Peumans P, Yakimov A, Forrest SR (2003) Small molecular weight organic thin-film photodetectors and solar cells. J Appl Phys 93:3693–3723
Ibn-Elhaj M, Schadt M (2001) Optical polymer thin films with isotropic and anisotropic nano-corrugated surface topologies. Nature 410:796–799
Chou SY, Krauss PR, Renstrom PJ (1995) Imprint of sub-25 nm vias and trenches in polymers. Appl Phys Lett 67:3114–3116
Buchko CJ, Kozloff KM, Martin DC (2001) Surface characterization of porous, biocompatible protein polymer thin films. Biomaterials 22:1289–1300
Vendra VK, Wu L, Krishnan S (2007) Polymer thin films for biomedical applications. Nanotechnologies for the Life Sciences. Wiley-VCH, Weinheim
Vázquez E, Dewitt DM, Hammond PT, Lynn DM (2002) Construction of hydrolytically-degradable thin films via layer-by-layer deposition of degradable polyelectrolytes. J Am Chem Soc 124:13992–13993
Yan W, Hsiao VKS, Zheng YB, Shariff YM, Gao T, Huang TJ (2009) Towards nanoporous polymer thin film-based drug delivery systems. Thin Solid Films 517:1794–1798
Joseph Kline R, McGehee MD, Toney MF (2006) Highly oriented crystals at the buried interface in polythiophene thin-film transistors. Nat Mater 5:222–228
Erbil HY, Demirel AL, Avci Y, Mert O (2003) Transformation of a simple plastic into a superhydrophobic surface. Science 299:1377–1380
Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE (2001) Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 70:1–20
Reynolds CH, Annan N, Beshah K, Huber JH, Shaber SH, Lenkinski RE, Wortman JA (2000) Gadolinium-loaded nanoparticles: new contrast agents for magnetic resonance imaging. J Am Chem Soc 122:8940–8945
You CC, Miranda OR, Gider B, Ghosh PS, Kim IB, Erdogan B, Krovi SA, Bunz UH, Rotello VM (2007) Detection and identification of proteins using nanoparticle-fluorescent polymer ‘chemical nose’ sensors. Nat Nanotechnol 2:318–323
Zhang Y, Wang L, Tian J, Li H, Luo Y, Sun X (2011) Ag@Poly(m-phenylenediamine) core-shell nanoparticles for highly selective, multiplex nucleic acid detection. Langmuir 27:2170–2175
Champion JA, Katare YK, Mitragotri S (2007) Particle shape: a new design parameter for micro- and nano-scale drug delivery carriers. J Control Release 121:3–9
Dean STH, William DR, Robert L (1983) Zero-order controlled-release polymer matrices for micro- and macromolecules. J Pharm Sci 72:17–22
Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE (2007) Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2:249–255
Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM (2008) The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–11618
Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR (2008) Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther 16:1450–1458
Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S (2009) Red blood cell-mimicking synthetic biomaterial particles. Proc Natl Acad Sci USA 106:21495–21499
Gopal R, Kaur S, Ma Z, Chan C, Ramakrishna S, Matsuura T (2006) Electrospun nanofibrous filtration membrane. J Membr Sci 281:581–586
Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253
Chand S (2000) Review Carbon fibers for composites. J Mater Sci 35:1303–1313. doi:10.1023/A:1004780301489
Khil MS, Cha DI, Kim HY, Kim IS, Bhattarai N (2003) Electrospun nanofibrous polyurethane membrane as wound dressing. J Biomed Mat Res B 67B:675–679
Kenawy ER, Bowlin GL, Mansfield K, Layman J, Simpson DG, Sanders EH, Wnek GE (2002) Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release 81:57–64
Naveen N, Kumar R, Balaji S, Uma TS, Natrajan TS, Sehgal PK (2010) Synthesis of nonwoven nanofibers by electrospinning: a promising biomaterial for tissue engineering and drug delivery. Adv Eng Mater 12:B380–B387
Vasita R, Katti DS (2006) Nanofibers and their applications in tissue engineering. Int J Nanomed 1(1):15–30
Sombatmankhong K, Suwantong O, Waleetorncheepsawat S, Supaphol P (2006) Electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate), and their blends. J Pol Sci B 44:2923–2933
Moroni L, Licht R, de Boer J, de Wijn JR, van Blitterswijk CA (2006) Fiber diameter and texture of electrospun PEOT/PBT scaffolds influence human mesenchymal stem cell proliferation and morphology, and the release of incorporated compounds. Biomaterials 27:4911–4922
Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS (2007) Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech 40:1686–1693
Yang F, Murugan R, Wang S, Ramakrishna S (2005) Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26:2603–2610
Yoon YI, Moon HS, Lyoo WS, Lee TS, Park WH (2008) Superhydrophobicity of PHBV fibrous surface with bead-on-string structure. J Colloid Interf Sci 320:91–95
Geoffrey T (1964) Disintegration of water drops in an electric field. Proc R Soc Lond A Mat 280:383–397
Rayleigh L (1882) On the equilibrium of liquid conducting masses charged with electricity. Phil Mag 14:184–186
Reneker DH, Yarin AL, Fong H, Koombhongse S (2000) Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys 87:4531–4547
Chen GQ, Wu Q (2005) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
Choi JS, Lee SW, Jeong L, Bae SH, Min BC, Youk JH, Park WH (2004) Effect of organosoluble salts on the nanofibrous structure of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Int J Biol Macromol 34:249–256
Gianino C (2006) Measurement of surface tension by the dripping from a needle. Phys Educ 41:440
Frost K, Kaminski D, Kirwan G, Lascaris E, Shanks R (2009) Crystallinity and structure of starch using wide angle X-ray scattering. Carbohydr Polym 78:543–548
Correia DM, Ribeiro C, Ferreira JCC, Botelho G, Ribelles JLG, Lanceros-Méndez S, Sencadas V (2013) Influence of electrospinning parameters on poly(hydroxybutyrate) electrospun membranes fiber size and distribution. Polym Eng Sci. doi:10.1002/pen.23704
Vehring R (2008) Pharmaceutical particle engineering via spray drying. Pharm Res 25:999–1022
Martin MA, Miguens FC, Rieumont J, Sanchez R (2000) Tailoring of the external and internal morphology of poly-3-hydroxy butyrate microparticles. Colloid Surf B 17:111–116
Li D, Marquez M, Xia Y (2007) Capturing electrified nanodroplets under Rayleigh instability by coupling electrospray with a sol-gel reaction. Chem Phys Lett 445:271–275
Almería B, Deng W, Fahmy TM, Gomez A (2010) Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. J Colloid Interf Sci 343:125–133
Xie J, Lim LK, Phua Y, Hua J, Wang CH (2006) Electrohydrodynamic atomization for biodegradable polymeric particle production. J Colloid Interf Sci 302:103–112
Fong H, Chun I, Reneker DH (1999) Beaded nanofibers formed during electrospinning. Polymer 40:4585–4592
Lertviriyasawat S, Chuachamsai A, Danwanichakul P (2008) Quantitative representation of chitosan/poly vinyl alcohol electrospun nanofiber morphology. Int J Sci Tech 13:48–53
Park WH, Jeong L, Yoo DI, Hudson S (2004) Effect of chitosan on morphology and conformation of electrospun silk fibroin nanofibers. Polymer 45:7151–7157
Wu X, Wang L, Yu H, Huang Y (2005) Effect of solvent on morphology of electrospinning ethyl cellulose fibers. J Appl Pol Sci 97:1292–1297
Reneker DH, Yarin AL (2008) Electrospinning jets and polymer nanofibers. Polymer 49:2387–2425
Sombatmankhong K, Sanchavanakit N, Pavasant P, Supaphol P (2007) Bone scaffolds from electrospun fiber mats of poly(3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and their blend. Polymer 48:1419–1427
Acknowledgements
The authors would like to thank Indian Council of Medical Research (ICMR), Department of Biotechnology (DBT), India, and Indian Institute of Technology Kanpur (IITK) for financial support; Advance Centre of Material Sciences (ACMS), Samtel Centre for Display Technology (SCDT) and Nanoscience Centre, IIT Kanpur for SEM facilities; Prof. R.N. Mukharjee, Department of Chemistry, IIT Kanpur for conductivity studies; Mr. U. S. Singh, ACMS, IIT Kanpur, for XRD studies; Prof. R. P. Singh, HBTI, Kanpur for surface tension studies (Tensiometer), Mr. Ayan Ray and Mr. Deepak Kumar for surface tension analysis by dripping from a needle method, and Mr. R. K. Verma for his help with determination of degree of crystallinity. BM would like to thank DBT and Council of Scientific and Industrial Research (CSIR), India for her research fellowship. DSK would like to acknowledge the “Batch of 1970 Research Fellowship” received from IITK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahaling, B., Katti, D.S. Fabrication of micro-structures of poly [(R)-3-hydroxybutyric acid] by electro-spraying/-spinning: understanding the influence of polymer concentration and solvent type. J Mater Sci 49, 4246–4260 (2014). https://doi.org/10.1007/s10853-014-8120-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8120-8