Abstract
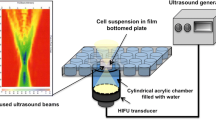
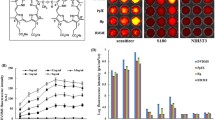
Low-energy ultrasound (LEUS) shows distinct potential as a safe therapeutic strategy for cancer treatment. Herein, mesoporous silica nanoparticles with closed-end cavities as sensitive nanoagents are prepared for effective cancer cell killing, when synergistically combined with mild LEUS (1 MHz, ≤1.0 W cm−2). The closed-end cavities can entrap gas bubbles, and provide a large number of cavitation nucleation sites, which could lead to drastically amplify ultrasonic cavitation effect by responding to the mild LEUS (1 MHz, ≤1.0 W cm−2). Significant killing effect against cancer cells is observed, when cells are treated by synergetic combination of mild LEUS and the nanoagents with closed-end cavities, showing distinct dose dependency on the nanoagents and irradiation intensity. Nevertheless, the killing effect is disappeared when the closed-end cavities are destructed. Moreover, no obvious cytotoxicity is observed when either the nanoagents or the LEUS is applied alone. The research may open up application opportunities of mild low-energy ultrasound for cancer therapy.





Similar content being viewed by others
References
Miller MW, Miller DL, Brayman AA (1996) A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol 22:1131–1154
Wang X, Chen HR, Zheng YY et al (2013) Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials 34:2057–2068
Suslick KS (1990) Sonochemistry. Science 247:1439–1445
Riesz P, Kondo T (1992) Free-radical formation induced by ultrasound and its biological implications. Free Radicl Bio Med 13:247–270
Jones SF, Evans GM, Galvin KP (1999) Bubble nucleation from gas cavities—a review. Adv Colloid Interface Sci 80:27–50
Holland CK, Apfel RE (1990) Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am 88:2059–2069
Man J, Shelton RM, Cooper PR, Landini G, Scheven BA (2012) Low intensity ultrasound stimulates osteoblast migration at different frequencies. J Bone Miner Metab 30:602–607
Wagstaffe SJ, Schiffter HA, Arora M, Coussios CC (2012) Sonosensitive nanoparticles for controlled instigation of cavitation and drug delivery by ultrasound. AIP Conf Proc 1481:426–431
Harle J, Mayia F, Olsen I, Salih V (2005) Effects of ultrasound on transforming growth factor-beta genes in bone cells. Eur Cells Mater 10:70–76
Reher P, Doan N, Bradnock B, Meghji S, Harris M (1999) Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine 11:416–423
terHaar G (2007) Therapeutic applications of ultrasound. Prog Biophys Mol Biol 93:111–129
Park K, Hoffmeister B, Han DK, Hasty K (2007) Therapeutic ultrasound effects on interleukin-1 beta stimulated cartilage construct in vitro. Ultrasound Med Biol 33:286–295
Pounder NM, Harrison AJ (2008) Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics 48:330–338
Lejbkowicz F, Salzberg S (1997) Distinct sensitivity of normal and malignant cells to ultrasound in vitro. Environ Health Perspect 105:1575–1578
Schuster A, Schwab T, Bischof M et al (2013) Cell specific ultrasound effects are dose and frequency dependent. Ann Anat 195:57–67
Wang Y, Bai WK, Shen E, Hu B (2013) Sonoporation by low-frequency and low-power ultrasound enhances chemotherapeutic efficacy in prostate cancer cells in vitro. Oncol Lett 6:495–498
Demos SM, Alkan-Onyuksel H, Kane BJ et al (1999) In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J Am Coll Cardiol 33:867–875
Ward M, Wu JR, Chiu JF (1999) Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents. J Acoust Soc Am 105:2951–2957
Feril LB, Kondo T, Zhao QL et al (2003) Enhancement of ultrasound-induced apoptosis and cell lysis by echo-contrast agents. Ultrasound Med Biol 29:331–337
Ju HY, Roy RA, Murray TW (2013) Gold nanoparticle targeted photoacoustic cavitation for potential deep tissue imaging and therapy. Biomed Opt Express 4:66–76
Sazgarnia A, Shanei A, Taheri AR et al (2013) Therapeutic effects of acoustic cavitation in the presence of gold nanoparticles on a colon tumor model. J Ultras Med 32:475–483
Zhao Y, Zhu YC, Fu JK, Wang LZ (2013) Effective cancer cell killing by hydrophobic nanovoid-enhanced cavitation under safe low-energy ultrasound. Chem Asian J. doi:10.1002/asia.201301333
de Sousa A, Maria DA, de Sousa RG, de Sousa EMB (2010) Synthesis and characterization of mesoporous silica/poly(N-isopropylacrylamide) functional hybrid useful for drug delivery. J Mater Sci 45:1478–1486
Zhu YC, Fujiwara M (2007) Installing dynamic molecular photomechanics in mesopores: a multifunctional controlled-release nanosystem. Angew Chem Int Ed 46:2241–2244
Chen F, Zhu YC (2012) Chitosan enclosed mesoporous silica nanoparticles as drug nano-carriers: sensitive response to the narrow pH range. Microporous Mesoporous Mater 150:83–89
Han LB, Zhou Y, He T et al (2013) One-pot morphology-controlled synthesis of various shaped mesoporous silica nanoparticles. J Mater Sci 48:5718–5726
Lang Y, Finn DP, Pandit A, Walsh PJ (2012) Pharmacological activity of ibuprofen released from mesoporous silica. J Mater Sci Mater Med 23:73–80
Rosenholm JM, Sahlgren C, Linden M (2010) Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles—opportunities and challenges. Nanoscale 2:1870–1883
Tsai CP, Chen CY, Hung Y, Chang FH, Mou CY (2009) Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J Mater Chem 19:5737–5743
Ferris DP, Lu J, Gothard C et al (2011) Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small 7:1816–1826
Rosenholm J, Sahlgren C, Linden M (2010) Cancer-cell targeting and cell-specific delivery by mesoporous silica nanoparticles. J Mater Chem 20:2707–2713
Chen Y, Chu C, Zhou Y et al (2011) Reversible pore-structure evolution in hollow silica nanocapsules: large pores for siRNA delivery and nanoparticle collecting. Small 7:2935–2944
Pesse AV, Warrier GR, Dhir VK (2005) An experimental study of the gas entrapment process in closed-end microchannels. Int J Heat Mass Transfer 48:5150–5165
Chappell MA, Payne SJ (2007) The effect of cavity geometry on the nucleation of bubbles from cavities. J Acoust Soc Am 121:853–862
Gelderblom H, Zijlstra AG, van Wijngaarden L, Prosperetti A (2012) Oscillations of a gas pocket on a liquid-covered solid surface. Phys Fluids. doi:10.1063/1.4769179
Borkent BM, Gekle S, Prosperetti A, Lohse D (2009) Nucleation threshold and deactivation mechanisms of nanoscopic cavitation nuclei. Phys Fluids. doi:10.1063/1.3249602
Mark G, Tauber A, Rudiger LA et al (1998) OH-radical formation by ultrasound in aqueous solution-Part II: terephthalate and Fricke dosimetry and the influence of various conditions on the sonolytic yield. Ultrason Sonochem 5:41–52
Saran M, Summer KH (1999) Assaying for hydroxyl radicals: hydroxylated terephthalate is a superior fluorescence marker than hydroxylated benzoate. Free Radic Res 31:429–436
Marschall HB, Morch KA, Keller AP, Kjeldsen M (2003) Cavitation inception by almost spherical solid particles in water. Phys Fluids 15:545–553
Feril LB, Kondo T (2004) Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound. J Radiat Res 45:479–489
Mitragotri S (2005) Innovation-Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov 4:255–260
Tang F, Li L, Chen D (2012) Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 24:1504–1534
Lu J, Liong M, Li Z, Zink JI, Tamanoi F (2010) Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 6:1794–1805
Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P (2013) Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev 42:1147–1235
Kroemer G, Jaattela M (2005) Lysosomes and autophagy in cell death control. Nat Rev Cancer 5:886–897
Chung MF, Chen KJ, Liang HF et al (2012) A liposomal system capable of generating CO2 bubbles to induce transient cavitation, lysosomal rupturing, and cell necrosis. Angew Chem Int Ed 51:10089–10093
Acknowledgements
The authors acknowledges financial support from the Nation Natural Science Foundation of China (51072217), the Science and Technology Commission of Shanghai (11XD1405600), the National High Technology Research and Development Program of China (2008AAO3Z303), the State Key Lab of High Performance Ceramics and Superfine Microstructure.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Zhu, Y. Synergistic cytotoxicity of low-energy ultrasound and innovative mesoporous silica-based sensitive nanoagents. J Mater Sci 49, 3665–3673 (2014). https://doi.org/10.1007/s10853-014-8073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-014-8073-y