Abstract
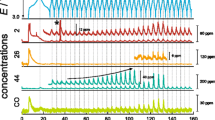
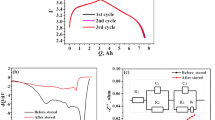
The evolution of gas in lithium-ion batteries (LIBs) at a charged state is one of the main problems in the industry because it causes significant distortion or swelling of the batteries. The mechanism of the gas-generating reaction related to the cathode at a charged state of LIBs was investigated. A side reaction between the electrolyte solution and free lithium compounds, such as Li2CO3 or LiOH in the cathode, is considered as the main cause of gas evolution at early stages of the storage test. Both Li2CO3 and LiOH generated CO2 mainly by a HF-mediated reaction, but the evolution of CO2 could be triggered by addition of H2O without a fluorine source for LiOH. Ni-based cathode materials generated more gas than conventional LiCoO2 at the initial stage because they contain more free lithium compounds, but the rate of gas evolution slowed down with time, suggesting that Ni-based active materials might be more appropriate for reducing long-term gas evolution in LIBs if the free lithium compounds could be removed effectively from the surface.


Similar content being viewed by others
References
Dominey LA (1994) Lithium batteries. Elsevier Science, Netherlands
Nazri GA, Pistoia G (2004) Lithium batteries: science and technology. Kluwer Academic Publishers, Boston
Yoshizawa H, Ohzuku T (2007) J Power Sources 174:813
Kim Y (2012) ACS Appl Mater Interfaces 4:2329
Kim Y (2013) J Solid State Electrochem 17:1961
Kim Y (2013) Phys Chem Chem Phys 15:6400
Kumai K, Miyashiro H, Kobayashi Y, Takei K, Ishikawa R (1999) J Power Sources 81:715
Kong W, Li H, Huang X, Chen L (2005) J Power Sources 142:285
Holzapfel M, Würsig A, Scheifele W, Vetter J, Novák P (2007) J Power Sources 174:1156
Kim Y, Kim D, Kang S (2011) Chem Mater 23:5388
Kim Y, Lee H, Kang S (2012) J Mater Chem 22:12874
van Schalkwijk W, Scrosati B (2002) Advances in lithium-ion batteries. Kluwer Academic/Plenum Publishers, New York
Aurbach D, Markovsky B, Shechter A, EinEli Y, Cohen H (1996) J Electrochem Soc 143:3809
Zhuang GV, Yang H, Ross PN, Xu K, Jow TR (2006) Electrochem Solid State Lett 9:A64
Du Pasquier A, Disma F, Bowmer T, Gozdz AS, Amatucci G, Tarascon JM (1998) J Electrochem Soc 145:472
Acknowledgements
This work was supported by Inha University Research Grant (INHA-46435).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y. Mechanism of gas evolution from the cathode of lithium-ion batteries at the initial stage of high-temperature storage. J Mater Sci 48, 8547–8551 (2013). https://doi.org/10.1007/s10853-013-7673-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7673-2