Abstract
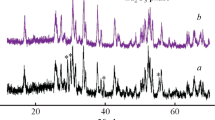
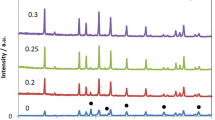
Cubic garnet of nominal composition Li7La3Zr2O12 (LLZO) is of interest as a possible electrolyte in a Li–metal halide battery composed of molten Li and an insoluble metal halide as electrodes operating in the temperature range of ~300–350 °C. As a consequence, the chemical stability of LLZO with molten Li in this temperature range was investigated. After heating in molten Li, the LLZO surface had undergone chemical coloration. X-diffraction, X-ray photoemission spectroscopy, electron paramagnetic resonance, and heat-treatment experiments suggest that chemical coloration is a result of the formation of color centers, composed of an electron trapped at an oxygen vacancy. It was also observed that after immersion in molten Li that LLZO exhibited intergranular cracking. It is believed that cracking results from stresses generated from an increased Li+ content, gained during immersion.





Similar content being viewed by others
References
Okamoto H (1995) J Phase Equilib 16:94
Murugan R, Thangadurai V, Weppner W (2007) Angew Chem Int Ed 46:7778
Kotoboki M, Kanamura K, Sato S, Yoshida T (2011) J Power Sources 196:7750
Rangasamy E, Wolfenstine J, Sakamoto J (2012) Solid State Ionics 206:28
Geiger CA, Alekseev E, Lazic B, Fisch M, Armbruster T, Langner R, Fechtelkord M, Kim N, Pettke T, Weppner W (2011) Inorg Chem 50:1089
Ni JE, Case ED, Sakamoto J, Rangasamy E, Wolfenstine J (2012) J Mater Sci 47:7978
Viswanathan L, Virkar AV (1982) J Mater Sci 17:753
Reed D, Coffey G, Mast E, Canfield J, Mansurov J, Lu X, Spenkle V (2013) J Power Sources 227:94
Weber N (1974) Energ Convers 14:1
De Jonghe LC, Buechele A (1982) J Mater Sci 17:885
De Jonghe LC, Buechele A, Armand M (1983) Solid State Ionics 9&10:165
Smaga JA, Battles J (1985) J Mat Sci Lett 4:553
Ansell RO (1986) J Mater Sci 21:365
Barsum M (1990) J Mater Sci 25:4393
Scmid H, De Jonghe LC, Cameron C (1982) Solid State Ionics 6:57
De Jonghe LC (1986) Ceram Bull 8:1158
Demott DS, Redfern BAW (1976) J Physique C 37:423
Kingery WD, Bowen HK, Uhlmann DR (1975) Introduction to ceramics. Wiley, New York
Barsum M (1997) Fundamentals of ceramics. The McGraw-Hill Company, New York
Mah T-I, Parthasarathy TA, Lee HD (2004) J Ceram Process Res 5:369
Mezeix L, Green DJ (2006) Int J Appl Ceram Tech 3:166
Yde-Anderson S, Lundsgaard JS, Moller L, Engell J (1984) Solid State Ionics 14:73
Gordon RS, Miller GR, McEntire BJ, Beck ED, Rasmussen JR (1981) Solid State Ionics 3(4):243
Jackman SD, Cutler RA (2012) J Power Sources 218:65
Lu X, Xia G, Lemmon JP, Yang Z (2010) J Power Sources 195:2431
Acknowledgements
JW, JAL, JR would like to acknowledge support of the Army Research Laboratory. JS would like to acknowledge the support of the US Army Research Office (ARO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolfenstine, J., Allen, J.L., Read, J. et al. Chemical stability of cubic Li7La3Zr2O12 with molten lithium at elevated temperature. J Mater Sci 48, 5846–5851 (2013). https://doi.org/10.1007/s10853-013-7380-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7380-z