Abstract
Silver compounds and silver ions are used extensively in medical devices because of their wide-spectrum antimicrobial activity. In particular, nanoparticles of silver and silver (I) oxide show great promise for widespread usage in medical polymers and nanodrugs. Here, we demonstrate that a crystalline powder and a saturated aqueous solution of silver (III) oxide clathrate show much stronger antimicrobial activities and oxidative activities than silver (I) oxide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silver compounds and silver ions exhibit antimicrobial properties [1–5]. They have a toxic effect on bacteria, viruses, and fungi, which is typical of heavy metals such as mercury, cadmium, and lead. However, in humans, they do not show the high levels of toxicity that are usually associated with other heavy metals. Since World War I, silver compounds have been used to prevent infection. For example, silver sulfadiazine cream has broad antimicrobial activity and is commonly used for burn wounds [2, 3].
Although the use of silver compounds reduced after the introduction of antibiotics, the evolution of antibiotic-tolerant bacteria, such as multi-drug resistant bacteria, has prompted a need to re-evaluate treatment strategies. In addition to the development of new medical devices, there is widespread use of silver alloy-coated urinary catheters, endotracheal breathing tubes and glass products coated with silver, silver compounds or silver-containing nanoparticles and glasses [6–10].
There are three oxidative forms of silver oxide: silver (I), Ag2O; silver (II or I, III), AgO; and silver (III), Ag2O3·Ag2O (I) is the most common oxide and it is used in the production of certain medical devices. AgO is part of the manufacture of silver oxide-zinc alkaline batteries and is formulated as Ag2O·Ag2O3. It has been reported that Ag2O3 can be isolated by electrolysis of NaClO4 and AgClO4 [11]. Although AgClO4 is a useful source of Ag+ ions, the presence of perchlorate represents human health and explosion risks. Pure Ag2O3 may be difficult to obtain commercially and industrially, and its therapeutic characteristics remain poorly understood.
In this study, we have identified a silver crystal which was produced by anodic oxidation of silver salts in aqueous solutions including AgNO3. We also performed to analyze the crystal with X-ray diffraction (XRD), and evaluate its antimicrobial activity.
Materials and methods
Preparation and identification of Ag2O3 clathrate
To obtain Ag2O3 clathrate, electrolysis of 100 mL of 1 M AgNO3 were carried out in 100-mL beaker with platinum electrodes, one of which was installed into a 35-mm film plastic case with two slits (40 × 5 mm2) as anode. Ag2O3 clathrate and pure silver were deposited on the anode and cathode, respectively. The plastic case prevents electrical short circuit caused by connection of either product on the electrodes. Approximately, 380 mg Ag2O3 clathrate was obtained in the plastic case of the electrolysis at 5 V dc for 15 min. XRD analysis of the Ag2O3 clathrate was performed with MO3XHF22 (MAC Science: X-rays Cu Kα: λ = 1.5406 Å, 40 kV, 30 mA, Ni filter and scan area: 10°≦ 2θ ≦ 70°, 2θ = 0.02).
Measurements of antimicrobial activity
Escherichia coli K-12 wild-type W3110 strain was used for measurement of antimicrobial activity of silver compounds. To suppress the precipitation of AgCl, we used a bacterial culture broth LB excluding NaCl for the bioassay. Radius of inhibitory zone of bacteria growth and area of compounds were measured by a stereomicroscopy with digital camera (Olympus) and Image J software (National Institute of Health, USA).
Results and discussion
In an earlier report, Ag2O3 crystal could be produced by anodic oxidation of silver salts in aqueous solutions including AgNO3 [12]. The crystal shows a cubic face centered oxide phase of the “ideal and stable composition” but it also contains Ag3+ and Ag+ ions in various proportions, as Ag2O3 clathrate [13].
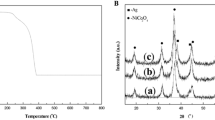
To confirm crystallizing Ag2O3 clathrate, electrolysis of the AgNO3 were performed, and resulted in the deposition of silver oxide and pure silver on the anode and cathode, respectively. The silver compound on the anode formed shiny black, needle-like crystals (Fig. 1). After washing the crystals several times with distilled water, XRD analysis indicated the presence of a single compound, which was identified as silver (III) oxide, i.e., Ag2O3 (Fig. 2). In addition, AgCl precipitation reaction with reactions of excess Cl− ions was used to estimate the concentration of Ag+ ions in the saturated solutions. The Ag+ ion concentration in the saturated water solution was 7.26 mM and much higher than that of Ag2O solution (0.40 mM) (Fig. 3). These results well confirmed that the shiny black compound is Ag2O3 clathrate.
Electrolysis of silver nitrate generates shiny black crystals of silver (III) oxide (Ag2O3) clathrate. Platinum electrodes were submerged in 1-M silver nitrate solution and electrolysis was performed using 5 V for 15–30 min. Approximately, 2.8% of the silver was recovered around the anode. Scale bar represents 5 mm
Solubility of Ag2O3 clathrate in Milli-Q water. a Excess NaCl solution was added to the supernatants of Ag2O and Ag2O3 clathrate saturated in Milli-Q water. White precipitates, AgCl appeared for the Ag2O3 clathrate solution (bottom). b Precipitation of silver oxide solutions. Calibration curve of the precipitation of 1× Ag2O solution and a 1/10 dilution of Ag2O3 solution were plotted with AgNO3 and excess NaCl standards. The measurement was triplicated and data represents the mean + standard error (SE)
To study its antimicrobial activity, 100 mg of Ag2O3 clathrate powder was spread onto an agar plate. An untreated control plate and silver-treated plate were then exposed to the environment, whereby the lids were removed and the plates left open to the air at room temperature. After more than a week, it was clear that the Ag2O3 clathrate had completely inhibited the growth of bacteria, fungi and myxomycetes, which were present on the untreated control plate (Fig. 4a). In addition, the presence of a small amount of Ag2O3 clathrate solid inhibited the growth of E. coli K-12 much more effectively than an equivalent amount of Ag2O (Fig. 4b, c). A saturated solution of Ag2O3 clathrate was prepared in Milli-Q water and 1 μL of the Ag2O3 solution was applied to a lawn of E. coli cells. A cleared area was evident for 24 h of incubation. Moreover, the saturated solution could completely sterilize 3 × 106 E. coli cells/mL within 3 h. The antimicrobial activity of the Ag2O3 clathrate solution appeared to be approximately 10-fold greater than that of the Ag2O solution (Figs. 3, 4d).
Strong antimicrobial activity of Ag2O3 clathrate. a Inhibitory effect of Ag2O3 clathrate powder on the growth of environmental microorganisms. Agar plates were prepared with and without 100 mg Ag2O3 clathrate powder spread on the agar surface. The lids were removed and then the plates were incubated for more than 1 week at room temperature. b–d Effect of Ag2O and Ag2O3 clathrate on the growth of Escherichia coli K-12. b Powder (upper particle area ca. 30 μm in diameter; bottom particle area ca. 150 μm in diameter) was applied to 3 mL of soft agar containing 107 bacteria. Scale bars represent 50 μm. c Radius of inhibitory zone of bacteria growth and area of Ag2O and Ag2O3 clathrate compounds were measured and plotted. d Liquid (1 μL Ag oxide-saturated MilliQ water) was deposited on plates spread with 107 bacteria. Plates (9 cm in diameter) contained LB agar lacking NaCl. Plates with E. coli were cultured at 37 °C for 24 h
Although scientists do not fully understand how silver compounds function against microorganisms, one hypothesis suggests that the antimicrobial activity is due to the chemical properties of its ionized form, i.e., Ag+. In bacteria, it appears that the ions form strong molecular bonds with substances used for respiration and, in particular, with molecules containing sulfur, nitrogen and oxygen [4]. Ag2O3 clathrate also exhibits strong oxidizing activity, since it can bleach red food coloring (Fig. 5) and react strongly to ammonium hydroxide, producing oxygen bubbles. Ag2O3 clathrate crystals are stable and show no alteration to electronic conductivity after 30 h UV (254 nm) irradiation. Therefore, Ag2O3 clathrate shows better solubility in pure water than Ag2O, leading to a greater availability of Ag+ ions, and its oxidizing activities may also play a role in the strong antimicrobial activity observed.
In conclusion, these results clearly indicate that Ag2O3 clathrate may serve as a useful and potent antimicrobial agent. It can be used as both a solid and in a saturated aqueous solution. Since the antimicrobial activity of Ag2O3 clathrate is greater than that of Ag2O, it may be expected to replace the use of Ag2O in certain medical equipment. It may be particularly suited to applications that require the reduction of biofilm formation by methicillin-resistant Staphylococcus aureus [6]. In addition, the procedure described for the isolation of Ag2O3 clathrate crystals is simple and inexpensive, which should enable its use to become widespread.
References
Lansdown ABG (2010) Silver in healthcare: its antimicrobial efficacy and safety in use. RSC, Cambridge
Atiyeh BS, Costagliola M, Hayek SN, Dibo SA (2007) Burns 33:139
Chopra I (2007) J Antimicrob Chemother 59:587
Slawson RM, Van Dyke MI, Lee H, Trevors JT (1992) Plasmid 27:72
Chang TW, Weinstein L (1975) Antimicrob Agents Chemother 8:677
Jang CH, Park H, Cho YB, Choi CH (2010) J Laryngol Otol 124:594
Rutberg FG, Dubina MV, Kolikov VA, Moiseenko FV, Ignat’eva EV, Volkov NM, Snetov VN, Stogov AY (2008) Dokl Biochem Biophys 421:191
Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, Howdle SM, Bayston R, Brown PD, Winship PD, Reid HJ (2004) J Antimicrob Chemother 54:1019
Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD (1998) Am J Med 105:236
Ahmed AA, Ali AA, Mahmoud DA, El-Fiqi AM (2011) J Biomed Mater Res A 98:132
Fischer P, Jansen M, Zahurak SM (2007) In: Murphy DW, Interrante LV (eds) Inorganic syntheses: nonmolecular solids, vol 30. Wiley, Hoboken, p 50
Náray-Szabó I, Popp K (1963) Z Anorg Allg Chem 322:286
Gmelin Handbuch, System No. 61, part B1 (1971)
Acknowledgements
We thank the Technical Division, School of Engineering, Tohoku University for XRD analysis and Ms. M. Hasegawa and Ms. Y. Sakamoto, Miyagi prefectural Sendai Daini Senior High School for helpful discussions. This study was supported by a joint program for the ‘Exploring Germination and Growth’ program for young Scientists (EGGS) at Tohoku University.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Saaya Ando, Tomosato Hioki, and Takamichi Yamada contributed equally to this study.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ando, S., Hioki, T., Yamada, T. et al. Ag2O3 clathrate is a novel and effective antimicrobial agent. J Mater Sci 47, 2928–2931 (2012). https://doi.org/10.1007/s10853-011-6125-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-6125-0