Abstract
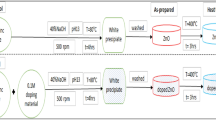
Zinc oxide (ZnO) micro-tubes via self-assembly of nanoparticles were synthesized by a simple precipitation process. Removal of As(III) (arsenite) from water by ZnO micro-tubes through adsorption was investigated with both lab-prepared and natural water samples. The result showed that these self-assembled ZnO micro-tubes are effective to remove As(III) from both lab-prepared and natural water samples at near neutral pH environment. These ZnO micro-tubes have a high adsorption capability on As(III) at low As(III) concentration. When the equilibrium As(III) concentration was around 0.1 mg/L, the amount of As(III) adsorbed at equilibrium was over 10 mg/g. At high equilibrium concentration, the adsorption capacity of these ZnO micro-tubes on As(III) reached over 39.4 mg/g. These ZnO micro-tubes could provide a simple single-step treatment option to treat arsenic-contaminated natural water, which requires no pre-treatment or post-treatment pH adjustment for current industrial practice.






Similar content being viewed by others
References
Nordstrom D (2002) Science 296(5576):2143
Amini M, Abbaspour K, Berg M, Winkel L, Hug S, Hoehn E, Yang H, Johnson C (2008) Environ Sci Technol 42(10):3669
Smith A, Lingas E, Rahman M (2000) Bull World Health Organ 78:1093
Hopenhayn-Rich C, Biggs M, Smith A (1998) Int J Epidemiol 27(4):561
Xia Y, Liu J (2004) Toxicology 198(1–3):25
Saha K (2003) Crit Rev Environ Sci Technol 33(2):127
Smith A, Lopipero P, Bates M, Steinmaus C (2002) Science 296(5576):2145
US EPA (2000) Technologies and costs for removal of arsenic from drinking water. US Environmental Protection Agency, Washington
Mohan D, Pittman C (2007) J Hazard Mater 142(1–2):1
Zhang G, Qu J, Liu H, Liu R, Li G (2007) Environ Sci Technol 41(13):4613
Borho M, Wilderer P (1996) Water Sci Technol 34(9):25
Lee H, Choi W (2002) Environ Sci Technol 36(17):3872
Kim Y, Kim C, Choi I, Rengaraj S, Yi J (2004) Environ Sci Technol 38(3):924
Edwards M (1994) J Am Water Works Assoc 86(9):64
McNeill L, Edwards M (1995) J Am Water Works Assoc 87(4):105
Hering J (1996) J Am Water Works Assoc 88(4):155
Pande S, Deshpande L, Patni P, Lutade S (1997) J Environ Sci Health Part A 32(7):1981
Özgür Ü, Alivov Y, Liu C, Teke A, Reshchikov M, Doğan S, Avrutin V, Cho S, Morkoc H (2005) J Appl Phys 98:041301
Meyer B, Alves H, Hofmann D, Kriegseis W, Forster D, Bertram F, Christen J, Hoffmann A, Straburg M, Dworzak M (2004) Phys Status Solidi (b) 241(2):231
Pan Z, Dai Z, Wang Z (2001) Science 291(5510):1947
Huang M, Mao S, Feick H, Yan H, Wu Y, Kind H, Weber E, Russo R, Yang P (2001) Science 292(5523):1897
Yang P, Yan H, Mao S, Russo R, Johnson J, Saykally R, Morris N, Pham J, He R, Choi H (2002) Adv Funct Mater 12(5):323
Tian Z, Voigt J, Liu J, Mckenzie B, Mcdermott M, Rodriguez M, Konishi H, Xu H (2003) Nat Mater 2(12):821
Iwasaki M, Inubushi Y, Ito S (1997) J Mater Sci Lett 16(18):1503
Jézéquel D, Guenot J, Jouini N, Fiévet F (1995) J Mater Res 10(1):77
Milosevic O, Uskokovic D (1993) Mater Sci Eng A 168(2):249
Chen D, Jiao X, Cheng G (1999) Solid State Commun 113(6):363
Li W, Shi E, Tian M, Zhong W (1998) Sci China Ser E Technol Sci 41(5):449
Zhang J, Sun L, Yin J, Su H, Liao C, Yan C (2002) Chem Mater 14(10):4172
Yao B, Chan Y, Wang N (2002) Appl Phys Lett 81:757
Yamabi S, Imai H (2002) J Mater Chem 12(12):3773
Hu J, Bando Y (2003) Appl Phys Lett 82:1401
Li Q, Kumar V, Li Y, Zhang H, Marks T, Chang R (2005) Chem Mater 17(5):1001
Hristovski K, Baumgardner A, Westerhoff P (2007) J Hazard Mater 147(1–2):265
Pena M, Korfiatis G, Patel M, Lippincott L, Meng X (2005) Water Res 39(11):2327
Barrett C, Massalski TB (1966) Structure of metals. McGraw Hill, New York
Sa Y, Aktay Y (2002) Biochem Eng J 12(2):143
Acknowledgements
This study was supported by the National Basic Research Program of China, Grant No. 2006CB601201, the Knowledge Innovation Program of Chinese Academy of Sciences, Grant No. Y0N5711171, and the Knowledge Innovation Program of Institute of Metal Research, Grant No. Y0N5A111A1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, W., Li, Q., Gao, S. et al. High efficient As(III) removal by self-assembled zinc oxide micro-tubes synthesized by a simple precipitation process. J Mater Sci 46, 5851–5858 (2011). https://doi.org/10.1007/s10853-011-5542-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-011-5542-4