Abstract
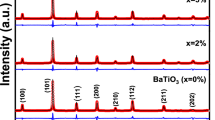
The stability of yttria-stabilized zirconia (YSZ) and scandia-stabilized zirconia (ScSZ) electrolytes against boron oxide was examined. Boron oxide was painted on the polished surface of YSZ and ScSZ and annealed at 1273 K for 100 h under wet hydrogen flowing condition. The X-ray diffractometry, scanning electron microscopy/energy dispersive X-ray analysis, and Raman studies revealed that formation of Y2O3 and Sc2O3 occurred on YSZ and ScSZ surfaces contacting the boron oxide, but rare earth borates were not observed. The surface of electrolytes around precipitated particles became rough and phase transformation was confirmed from the cubic to the tetragonal or the monoclinic phases due to stabilizer removal from cubic zirconia. It has been also verified that small amounts of zirconium and yttrium were transported from the electrolyte to the gas phase via boron component. This destabilization effect induced by boron oxide was more serious for ScSZ than for YSZ. A destabilization mechanism under wet hydrogen atmosphere is proposed based on pseudo ternary phase diagrams for the YO1.5–BO1.5–ZrO2 system and the ScO1.5–BO1.5–ZrO2 system and thermodynamic considerations.







Similar content being viewed by others
References
Abriata JP, Garces J, Versaci R (1986) Bull Alloy Phase Diagr 7:116
Wang WE, Olander DR (1993) J Nucl Mater 201:231
Minh NQ, Takahashi T (1995) In: Science and technology of ceramic fuel cells. Elsevier, Amsterdam, p 69
Ishihara T, Sammes NM, Yamamoto O (2003) In: Singhal SC, Kendall K (eds) High temperature solid oxide fuel cells—fundamentals, design and applications. Elsevier, Oxford, p 83
Mizutani Y, Tamura M, Kawai M, Yamamoto O (1994) Solid State Ion 72:271
Yokokawa H, Horita T, Yamaji K, Kishimoto H, Xiong YP, Brito ME (2008) In: Steinberger-Wilckens R (ed) Proceedings of 8th European solid oxide fuel cell forum, B1004
Toriz FC, Thakker AB, Gupta SK (1989) Surf Coat Technol 39/40:161
Susnitzky DW, Hertl W, Barry Carter C (1988) J Am Ceram Soc 71:992
Hertl W (1988) J Appl Phys 63:5514
Jones RL (1990) High Temp Sci 27:369
Yokokawa H, Sakai N, Kawada T, Dokiya M (1993) In: Badwal SPS, Bannister MJ, Hannink RHJ (eds) Science and technology of zirconia V. The Tenomic Publication Co. Inc., Lancaster, PA, USA, p 752
Buchanan RC, Sircar A (1983) Comm Am Ceram Soc Feb:C-20
Ananthapadmanabhan PV, Menon SB, Venkatramani N, Rohatgi VK (1989) J Mater Sci 24:4432
Guo X (1996) J Mater Sci Lett 15:38
de Florio DZ, Muccillo R (2004) Mater Res Bull 39:1539
Calculated with the thermodynamic database MALT for windows and the programs gem and CHD, Kagaku gijutsu-Sha, Japan. https://doi.org/ww.kagaku.com/malt/
Fujimori H, Yashima M, Kakihana M, Yoshimura M (1998) J Am Ceram Soc 81:2885
Levin EM (1967) J Am Ceram Soc 50:53
Figure 4390 (1975) In: Levin EM, McMurdie HF (eds) Phase diagrams for ceramists. The American Ceramic Society, Inc., p 140
Yokokawa H, Sakai N, Kawada T, Dokiya M (1993) In: Badwal SPS, Bannister MJ, Hannink RHJ (eds) Science and technology of zirconia V. Tenomic Publication Co. Inc., Lancaster, PA, USA, p 592
Acknowledgement
This study was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kishimoto, H., Sakai, N., Yamaji, K. et al. Destabilization of cubic-stabilized zirconia electrolyte induced by boron oxide under reducing atmosphere. J Mater Sci 44, 639–646 (2009). https://doi.org/10.1007/s10853-008-3061-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-3061-8