Abstract
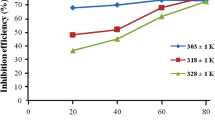
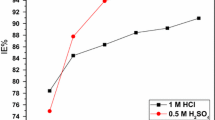
Exudate gum from Raphia hookeri (RH) was tested as corrosion inhibitor for mild steel in H2SO4 using weight loss and hydrogen evolution techniques at 30–60 °C. Results obtained revealed that RH act as corrosion inhibitor for mild steel in sulfuric acid medium. The corrosion rates in all concentrations studied increased with rise in temperature. The inhibition efficiency was observed to increase with increase in RH concentration but decreased with rise in temperature, which is suggestive of physical adsorption mechanism. The inhibitive action of RH is discussed in view of the adsorption of its phytochemical components onto steel surface, which protects the metal surface and thus do not permit the corrosion process to take place. The adsorption of the exudate gum onto the steel surface was found to follow the Langmuir adsorption isotherm. The free energies for the adsorption process and the apparent activation energies, enthalpies and entropies of the dissolution process were determined. The fundamental thermodynamic functions were used to glean important information about the RH inhibitory behavior. The results were explained in terms of chemical thermodynamics.





Similar content being viewed by others
References
El-Etre AY (2007) J Colloid Interface Sci 314:578
Loto CA, Mohammed AI (2000) Corros Prev Control 47(2):50
Gunasekeran G, Chauhan LR (2004) Electrochim Acta 49(25):4387
Kliskic M, Radosevic J, Gudic S, Katalinic V (2000) J Appl Electrochem 30:823
Avwiri GO, Igbo FO (2003) Mater Lett 57:3705
Martinez S, Stern I (2001) J Appl Electrochem 33:1137
Ashassi-Sorkhabi H, Seifzadeh D (2006) Int J Electrochem Sci 1:92
Umoren SA, Obot IB, Ebenso EE, Obi-Egbedi NO (2008) Port Electrochim Acta 26:199
Umoren SA, Obot IB, Ebenso EE (2008) E-J Chem 5(2):355
Umoren SA, Obot IB, Akpabio LE, Etuk SE (2008) Pigm Resin Technol 37(2):98
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Anti-Corros Methods Mater 53(5):277
Umoren SA, Ogbobe O, Ebenso EE (2006) Trans SAEST 41:74
Umoren SA, Ogbobe O, Ebenso EE, Ekpe UJ (2006) Pigm Resin Technol 35:284
Umoren SA, Ogbobe O, Ebenso EE (2006) Bull Electrochem 22:155
Ekpe UJ, Ebenso EE, Antia BS (1999) West Afr J Biol Appl Chem 41:16
Gomma GK (1998) Mater Chem Phys 55:241
Jones AD (1996) Principles and prevention of corrosion, 2nd edn. Printice Hall Inc., Upper Saddle River, NJ, p 31
Ebenso EE, Ibok UJ, Ekpe UJ, Umoren SA, Jackson E, Abiola OK, Oforka NC, Martinez S (2004) Trans SAEST 39:117
Onuchukwu AI (1988) Mater Chem Phys 20:323
Oguzie EE (2005) Pigm Resin Technol 34(6):321
Bouklah M, Hammouti B (2006) Port Electrochim Acta 24:457
Okafor PC, Ekpe UJ, Ebenso EE, Umoren EM, Leizou KE (2005) Bull Electrochem 8:347
Umoren SA, Ebenso EE (2007) Mater Chem Phys 106(2–3):387
Yurt A, Balaban A, Kandermir SU, Bereket G, Erk B (2004) Mater Chem Phys 85:420
Radovici O (1965) Proceedings of the 2nd European symposium on corrosion inhibition, Ferrara, Italy, p 178
Martinez S, Metikos-Hukovic M (2003) J Appl Electrochem 33:1137
Popova A, Sokolova E, Raicheva S, Christov M (2003) Corros Sci 45:33
Zucchi F, Trabanelli G, Brunoro G (1994) Corros Sci 36:1683
Bochris JOM, Reddy AKN (1977) Modern electrochemistry, vol 2. Plenum Press, New York, p 1267
Tang LB, Mu GN, Liu GH (2003) Corros Sci 45:2251
Bouklah M, Benchat N, Hammouti B, Aouniti A, Kertit S (2006) Mater Lett 60:1901
Bentiss F, Traisnel M, Lagrenee M (2000) Corros Sci 42:127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umoren, S.A., Obot, I.B. & Obi-Egbedi, N.O. Raphia hookeri gum as a potential eco-friendly inhibitor for mild steel in sulfuric acid. J Mater Sci 44, 274–279 (2009). https://doi.org/10.1007/s10853-008-3045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-3045-8