Abstract
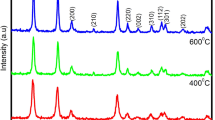
Tin oxide nanoparticles were synthesized by electrochemical oxidation of a tin metal sheet in a non-aqueous electrolyte containing NH4F. The as-prepared nanoparticles were then thermally annealed at 700 °C for 6 h. The resulting particles were characterized by a variety of experimental techniques, including X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), Raman, UV-visible, and photoluminescence (PL) spectroscopy. The XRD patterns clearly showed that the amorphous phase of the as-synthesized SnO2 particles was transformed into a rutile-type crystalline structure after thermal treatment; and from the line broadening of the XRD peaks, the average size of the annealed particles was found to be 15.4, 12.5, 11.8 nm for the particles initially synthesized at 20, 30, and 40 V, respectively. Consistent results were also observed in HRTEM measurements which showed clear crystalline lattice fringes of the calcined nanoparticles, as compared to the featureless profiles of the as-produced counterparts. In Raman spectroscopic studies, three dominant peaks were observed at 480, 640, and 780 cm−1 which were ascribed to the E1g, A1g, and B2g Raman active vibration modes, respectively, and the wavenumbers of these peaks blue-shifted with decreasing particle size. Additionally, a broad strong emission band was observed in room-temperature photoluminescence measurements.






Similar content being viewed by others
References
Ferrere S, Zaban A, Gregg BA (1997) J Phys Chem B 101:4490. doi:https://doi.org/10.1021/jp970683d
Wang Y, Lee JY, Zeng HC (2005) Chem Mater 17:3899. doi:https://doi.org/10.1021/cm050724f
Wang Y, Zeng HC, Lee JY (2006) Adv Mater 18:645. doi:https://doi.org/10.1002/adma.200501883
Stampfl SR, Chen Y, Dumesic JA, Niu CM, Hill CG (1987) J Catal 105:445. doi:https://doi.org/10.1016/0021-9517(87)90072-8
Zhang Y, Kolmakov A, Lilach Y, Moskovits M (2005) J Phys Chem B 109:1923. doi:https://doi.org/10.1021/jp045509l
Nicholas CP, Marks TJ (2004) Nano Lett 4:1557. doi:https://doi.org/10.1021/nl049255r
Wang YL, Jiang XC, Xia YN (2003) J Am Chem Soc 125:16176. doi:https://doi.org/10.1021/ja037743f
Comini E, Faglia G, Sberveglieri G, Pan ZW, Wang ZL (2002) Appl Phys Lett 81:1869. doi:https://doi.org/10.1063/1.1504867
Kolmakov A, Zhang YX, Cheng GS, Moskovits M (2003) Adv Mater 15:997. doi:https://doi.org/10.1002/adma.200304889
Law M, Kind H, Messer B, Kim F, Yang PD (2002) Angew Chem Int Ed 41:2405. doi:10.1002/1521-3773(20020703)41:13<2405::AID-ANIE2405>3.0.CO;2-3
Huang J, Matsunaga N, Shimanoe K, Yamazoe N, Kunitake T (2005) Chem Mater 17:3513. doi:https://doi.org/10.1021/cm047819m
Akari S, Friemelt K, Glockler K, Luxsteiner MC, Bucher E, Dransfeld K (1993) Appl Phys A Mater Sci Process 57:221
Tatsuyama C, Ichimura S (1976) Jpn J Appl Phys 15:843. doi:https://doi.org/10.1143/JJAP.15.843
Cheng Y, Xiong P, Fields L, Zheng JP, Yang RS, Wang ZL (2006) Appl Phys Lett 89:093114. doi:https://doi.org/10.1063/1.2338754
Yang YY, Pradhan S, Chen SW (2004) J Am Chem Soc 126:76. doi:https://doi.org/10.1021/ja037675x
Leite ER, Weber IT, Longo E, Varela JA (2000) Adv Mater 12:965. doi:10.1002/1521-4095(200006)12:13<965::AID-ADMA965>3.0.CO;2-7
Pang GS, Chen SG, Koltypin Y, Zaban A, Feng SH, Gedanken A (2001) Nano Lett 1:723. doi:https://doi.org/10.1021/nl0156181
Juttukonda V, Paddock RL, Raymond JE, Denomme D, Richardson AE, Slusher LE et al (2006) J Am Chem Soc 128:420. doi:https://doi.org/10.1021/ja056902n
Jiang LH, Sun GQ, Zhou ZH, Sun SG, Wang Q, Yan SY et al (2005) J Phys Chem B 109:8774. doi:https://doi.org/10.1021/jp050334g
Xu CK, Xu GD, Liu YK, Zhao XL, Wang GH (2002) Scr Mater 46:789. doi:https://doi.org/10.1016/S1359-6462(02)00077-5
Vayssieres L, Graetzel M (2004) Angew Chem Int Ed 43:3666. doi:https://doi.org/10.1002/anie.200454000
Liu YK, Zheng CL, Wang WZ, Yin CR, Wang GH (2001) Adv Mater 13:1883. doi:10.1002/1521-4095(200112)13:24<1883::AID-ADMA1883>3.0.CO;2-Q
Wang WZ, Xu CK, Wang GH, Liu YK, Zheng CL (2002) J Appl Phys 92:2740. doi:https://doi.org/10.1063/1.1497718
Duan JH, Yang SG, Liu HW, Gong JF, Huang HB, Zhao XN et al (2005) J Am Chem Soc 127:6180. doi:https://doi.org/10.1021/ja042748d
Ding ZF, Quinn BM, Haram SK, Pell LE, Korgel BA, Bard AJ (2002) Science 296:1293. doi:https://doi.org/10.1126/science.1069336
Lou XW, Wang Y, Yuan CL, Lee JY, Archer LA (2006) Adv Mater 18:2325. doi:https://doi.org/10.1002/adma.200600733
Yu JG, Guo HT, Davis SA, Mann S (2006) Adv Funct Mater 16:2035. doi:https://doi.org/10.1002/adfm.200600552
Zhu W, Wang WZ, Xu HL, Shi JL (2006) Mater Chem Phys 99:127. doi:https://doi.org/10.1016/j.matchemphys.2005.10.002
Xie J, Varadan VK (2005) Mater Chem Phys 91:274. doi:https://doi.org/10.1016/j.matchemphys.2004.11.033
Duan XF, Lieber CM (2000) Adv Mater 12:298. doi:10.1002/(SICI)1521-4095(200002)12:4<298::AID-ADMA298>3.0.CO;2-Y
Morales AM, Lieber CM (1998) Science 279:208. doi:https://doi.org/10.1126/science.279.5348.208
Wang B, Yang YH, Wang CX, Xu NS, Yang GW (2005) J Appl Phys 98:124303
Dai ZR, Gole JL, Stout JD, Wang ZL (2002) J Phys Chem B 106:1274. doi:https://doi.org/10.1021/jp013214r
Liu Y, Dong H, Liu ML (2004) Adv Mater 16:353. doi:https://doi.org/10.1002/adma.200306104
Mukhamedshina DM, Beisenkhanov NB, Mit KA, Valitova IV, Botvin VA (2005) High Temp Mater Process 9:307. doi:https://doi.org/10.1615/HighTempMatProc.v9.i2.130
Minami T, Nanto H, Takata S (1988) Jpn J Appl Phys Part 2 Lett 27:L287
Ye CH, Fang XS, Wang YH, Xie TW, Zhao AW, Zhang LD (2004) Chem Lett 33:54. doi:https://doi.org/10.1246/cl.2004.54
Han WQ, Zettl A (2003) Nano Lett 3:681. doi:https://doi.org/10.1021/nl034142d
Zhu HL, Yang DR, Yu GX, Zhang H, Yao KH (2006) Nanotechnology 17:2386. doi:https://doi.org/10.1088/0957-4484/17/9/052
Reetz MT, Helbig W (1994) J Am Chem Soc 116:7401. doi:https://doi.org/10.1021/ja00095a051
Reetz MT, Helbig W, Quaiser SA (1995) Chem Mater 7:2227. doi:https://doi.org/10.1021/cm00060a004
Talapin DV, Murray CB (2005) Science 310:86. doi:https://doi.org/10.1126/science.1116703
Dierstein A, Natter H, Meyer F, Stephan HO, Kropf C, Hempelmann R (2001) Scr Mater 44:2209. doi:https://doi.org/10.1016/S1359-6462(01)00906-X
Kamada K, Mukai M, Matsumoto Y (2002) Electrochim Acta 47:3309. doi:https://doi.org/10.1016/S0013-4686(02)00251-7
Ruan CM, Paulose M, Varghese OK, Mor GK, Grimes CA (2005) J Phys Chem B 109:15754. doi:https://doi.org/10.1021/jp052736u
Paulose M, Shankar K, Yoriya S, Prakasam HE, Varghese OK, Mor GK et al (2006) J Phys Chem B 110:16179. doi:https://doi.org/10.1021/jp064020k
Tsuchiya H, Macak JM, Taveira L, Balaur E, Ghicov A, Sirotna K et al (2005) Electrochem Commun 7:576. doi:https://doi.org/10.1016/j.elecom.2005.04.008
Yusta FJ, Hitchman ML, Shamlian SH (1997) J Mater Chem 7:1421. doi:https://doi.org/10.1039/a608525c
Maestre D, Ramirez-Castellanos J, Hidalgo P, Cremades A, Gonzalez-Calbet JM, Piqueras J (2007) Eur J Inorg Chem 1544. doi:https://doi.org/10.1002/ejic.200600990
Porto SPS, Fleury PA, Damen TC (1967) Phys Rev 154:522. doi:https://doi.org/10.1103/PhysRev.154.522
Trayler JG, Smith HG, Nicklow RM, Wilkinson MK (1971) Phys Rev B 3:3457. doi:https://doi.org/10.1103/PhysRevB.3.3457
Rumyantseva MN, Gaskov AM, Rosman N, Pagnier T, Morante JR (2005) Chem Mater 17:893. doi:https://doi.org/10.1021/cm0490470
Sun SH, Meng GW, Zhang GX, Gao T, Geng BY, Zhang LD et al (2003) Chem Phys Lett 376:103. doi:https://doi.org/10.1016/S0009-2614(03)00965-5
Peercy PS, Morosin B (1973) Phys Rev B 7:2779. doi:https://doi.org/10.1103/PhysRevB.7.2779
Parker JC, Siegel RW (1990) Appl Phys Lett 57:943. doi:https://doi.org/10.1063/1.104274
Aita CR (2007) Appl Phys Lett 90:213112. doi:https://doi.org/10.1063/1.2742914
Abello L, Bochu B, Gaskov A, Koudryavtseva S, Lucazeau G, Roumyantseva M (1998) J Solid State Chem 135:78. doi:https://doi.org/10.1006/jssc.1997.7596
Dieguez A, Romano-Rodriguez A, Vila A, Morante JR (2001) J Appl Phys 90:1550. doi:https://doi.org/10.1063/1.1385573
Cheng B, Russell JM, Shi WS, Zhang L, Samulski ET (2004) J Am Chem Soc 126:5972. doi:https://doi.org/10.1021/ja0493244
Hu JQ, Bando Y, Liu QL, Golberg D (2003) Adv Funct Mater 13:493. doi:https://doi.org/10.1002/adfm.200304327
Jeong J, Choi SP, Chang CI, Shin DC, Park JS, Lee BT et al (2003) Solid State Commun 127:595. doi:https://doi.org/10.1016/S0038-1098(03)00614-8
Yu D, Wang CJ, Wehrenberg BL, Guyot-Sionnest P (2004) Phys Rev Lett 92:216802. doi:https://doi.org/10.1103/PhysRevLett.92.216802
Wang B, Yang YH, Wang CX, Yang GW (2005) Chem Phys Lett 407:347. doi:https://doi.org/10.1016/j.cplett.2005.03.119
Acknowledgements
This work was supported in part by the National Science Foundation (CHE-0718170 and DMR-0804049). The powder X-ray diffraction data in this work were recorded on an instrument supported by the NSF Major Research Instrumentation (MRI) Program under Grant No. CHE-0521569. We thank the National Center for Electron Microscopy at Lawrence Berkeley National Laboratory for use of its facilities. We also thank Prof. J. Z. Zhang, T. Olson and R. Newhouse (UCSC) for access to the Raman spectrometer, and Prof. S. Oliver and D. Rogow (UCSC) for assistance in XRD data acquisition.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Ghosh, D. & Chen, S. Large-scale electrochemical synthesis of SnO2 nanoparticles. J Mater Sci 43, 5291–5299 (2008). https://doi.org/10.1007/s10853-008-2792-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2792-x