Abstract
Grain growth in two-dimensional polycrystals with mobile pores at the grain boundary triple junctions is considered. The kinetics of grain and pore growth are determined under the assumption that pore sintering and pore mobility are controlled by grain boundary and surface diffusion, respectively. It is shown that a polycrystal can achieve full density in the course of grain growth only when the initial pore size is below a certain critical value which depends on kinetic parameters, interfacial energies, and initial grain size. Larger pores grow without limits with the growing grains, and the corresponding grain growth exponent depends on kinetic parameters and lies between 2 and 4. It is shown that for a polycrystal with subcritical pores the average grain size increases linearly with time during the initial stages of growth, in agreement with recent experimental data on grain growth in thin Cu films and in bulk nanocrystalline Fe.





Similar content being viewed by others
References
Gleiter H (1989) Prog Mater Sci 33:223. doi:https://doi.org/10.1016/0079-6425(89)90001-7
Chaudhari PJ (1972) Vac Sci Technol 9:520. doi:https://doi.org/10.1116/1.1316674
Upmanyu M, Srolovitz DJ, Gottstein G, Shvindlerman LS (1998) Interface Sci 6:289. doi:https://doi.org/10.1023/A:1008653704896
Shvindlerman LS, Gottstein G, Ivanov VA, Molodov DA, Kolesnikov D, Lojkowski W (2006) J Mater Sci 41:7725. doi:https://doi.org/10.1007/s10853-006-0563-0
Brook RJ (1976) In: Wang FFY (eds) Treatise on materials science and technology, vol 9. Academic, New York, pp 331–363
Yan MF, Cannon RM, Chowdhry U (1978) Am Ceram Bull 57:316
Gottstein G, Shvindlerman LS (1993) Acta metal mater 41:3267
Gottstein G, Ma Y, Shvindlerman LS (2005) Acta mater 53:1535. doi:https://doi.org/10.1016/j.actamat.2004.12.006
Gottstein G, Shvindlerman LS (2006) Scripta mater 54:1065. doi:https://doi.org/10.1016/j.scriptamat.2005.11.057
Cahn JW (1962) Acta metal 10:789
Gottstein G, Shvindlerman LS (1999) Grain boundary migration in metals. CRC Press, Boca Raton
Spears MA, Evans AG (1982) Acta metal 30:1281
Riedel H, Svoboda J (1993) Acta metal mater 41:1929
Humphreys FJ, Hatherly M (1996) Recrystallization and related annealing phenomena. Elsevier, Oxford
Mishin Y, Kaur I, Gust W (1995) Fundamentals of grain and interphase boundary diffusion. Wiley, Chichester
Schönfelder B, Gottstein G, Shvindlerman LS (2005) Acta mater 53:1597. doi:https://doi.org/10.1016/j.actamat.2004.12.010
Sinclair CW, Hutchinson CR, Brechet Y (2007) Met Mater Trans A 38:821. doi:https://doi.org/10.1007/s11661-007-9106-9
Fradkov VE, Udler D (1994) Adv Phys 43:739. doi:https://doi.org/10.1080/00018739400101559
Cao P, Zhang B (2006) Int J Mod Phys B 20:3830. doi:https://doi.org/10.1142/S0217979206040441
Krill III CE, Helfen L, Michels D, Natter H, Fitch A, Masson O, Birringer R (2001) Phys Rev Lett 86:842. doi:https://doi.org/10.1103/PhysRevLett.86.842
Estrin Y, Gottstein G, Rabkin E, Shvindlerman LS (2000) Scripta mater 43:141. doi:https://doi.org/10.1016/S1359-6462(00)00383-3
Klinger L, Rabkin E, Shvindlerman LS, Gottstein G (2008) J Mater Sci, to be submitted
Acknowledgements
This work was supported by the Russell Berrie Nanotechnology Institute (Technion) and by the RWTH Aachen through the Umbrella Cooperation Program.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Dissolution of a circular pore at a triple junction
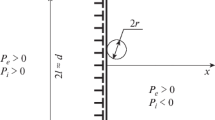
Let us consider a cylindrical pore of radius r located at a GB triple junction in a two-dimensional polycrystal. We will assume that pore dissolution is controlled by GB diffusion alone. The diffusion flux, j, along the GB (per unit length of the pore) is
where σnn is the normal stress at the GB, and x is the coordinate along the GB. D GB is the GB-self diffusion coefficient and we assume for simplicity that the diffusional width of the GB is equal to that of the surface. In the middle between two identical pores (x = 0) this flux should vanish because of symmetry reasons. In the steady state sintering regime the drift velocities of two grains normal to the GB should be constant, which means that the divergence of the flux j given by Eq. A1 is also constant. In this case
where a and b are constants. The distance between neighboring pores is 2l ≈ 2πL/n − 2r (see Fig. 1). The chemical potential of the atoms on the pore surface and at the triple junction between the pore surface and a GB are μ0 − γsΩ/r and μ0 + σnnΩ, respectively (here, γs is the surface energy and μ0 is the chemical potential of atoms in the crystal bulk). From the balance of these chemical potentials we get
To find the unknown constants a and b we use the boundary condition A3 and the total force balance at the GB:
which yields \(a=-\frac{3\gamma_s(l+r)}{2rl^3}.\) For the atomic flux to the pore we obtain from Eqs. A1 and A2
The rate of pore dissolution can be found from the condition of mass balance:
For l ≫ r
Rights and permissions
About this article
Cite this article
Klinger, L., Rabkin, E., Shvindlerman, L.S. et al. Grain growth in porous two-dimensional nanocrystalline materials. J Mater Sci 43, 5068–5075 (2008). https://doi.org/10.1007/s10853-008-2678-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2678-y