Abstract
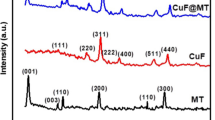
Multiwall carbon nanotubes have been grown on montmorillonite clay catalysts through anchoring on FeCo nanoparticles. The starting clay is a commercial sodium-rich montmorillonite in which the intercalated sodium ion was exchanged for cobalt(II) and iron(III) ions via mechanical agitation or sonication, both with and without subsequent centrifugation. The cobalt-iron intercalate clay was used as a catalyst for the synthesis of carbon nanotubes via decomposition of ethylene at 700 °C. The largest carbon deposit was obtained for catalysts prepared with 3 or 4 cation exchange equivalents. X-ray diffraction indicates both that the basal spacing of the clay increases from 12.43 Å to 16.4 Å upon intercalation of cobalt and iron. Atomic absorption analysis of the catalysts indicates that virtually all of the sodium ions originally present in the clay have been replaced by iron(III) and cobalt(II). Transmission electron micrographs show the presence of multiwall carbon nanotubes with inner and outer diameters of ca. 10 nm and 20 nm grown on metal particles present on the plates of catalysts. The iron-57 Mössbauer spectra indicate that the intercalated clay contains iron(III) in octahedral and tetrahedral sites and iron(II) in octahedral sites, the catalysts contain an extensive amount of small superparamagnetic particles of α-Fe2O3 and that the carbon-nanotube catalyst composites show the presence of iron(II) and iron(III) paramagnetic doublets, characteristic of a reduced montmorillonite, and of sextets that are characteristic of an FeCo alloy and of Fe3C cementite. The Mössbauer spectra indicate that the carbon nanotubes grow on FeCo metallic nanoparticles and bond to these particles through the formation of cementite.















Similar content being viewed by others
References
Hernadi K, Fonseca A, Nagy JB, Bernaerts D, Lucas AA (1996) Carbon 34:1249
Hernadi K, Kónya Z, Siska A, Kiss J, Oszkó A, Nagy JB, Kiricsi I (2003) Mater Chem Phys 77:536
Hernadi K (2002) Chem Phys Lett 363:169
Gournis D, Karakassides MA, Bakas T, Boukos N, Petridis D (2003) Carbon 40:2641
Jankovic L, Gournis D, Dimos K, Karakassides MA, Bakas T (2005) J Phys Conf Series 10:178
Gil A, Gandia LM, Vicente MA (2000) Catal Rev Sci Eng 42:212
Endo M, Takeuchi K, Igarashi S, Kobori K, Shiraishi M, Kroto HW (1993) J Phys Chem Solids 54:1841
Pinnavaia TJ, Tzou MS, Landau SD, Raythatha RH (1984) J Mol Catal 27:195
Weaver CE, Pollard LD (1973) The chemistry of clay minerals developments in sedimentology, vol 15. Elsevier, New York, p 213
Bakandritsos A, Simopoulos A, Petridis D (2006) Nanotechnology 17:1112
Moore DM, Reynolds RC (1989) X-ray diffraction and the identification and analysis of clay minerals. Oxford University Press, Oxford, p 332
Thorez J (1976) Practical identification of clay minerals. A handbook for teachers and students in clay mineralogy. Lelotte, Dison, Belgium, p 90
Velde B (1992) Introduction to the clay minerals. Chapman and Hall, London, p 198
Önal M, Sarikaya Y, Alemdaroglu T, Bozdogan I (2003) Turk J Chem 27:683
Girgis BS, El Barawy KA, Felix NS (1987) Thermochim Acta 111:9
Van Bekkum H, Flanigen EM, Jacobs PA, Jansen JC (eds) (2001) Studies in surface science and catalysis, vol 137. Elsevier, Amsterdam
Storaro L, Lenarda M, Ganzerla R, Rinaldi A (1996) Microporous Mater 6:55
Ebbinghaus SG, Mauron PH, Reller A, Zhang Y, Zuttel A (2002) Mater Sci Eng C 19:119
Nagy JB, Bister G, Fonseca A, Méhn D, Konya Z, Kiricsi I, Horvath ZE, Biro LP (2004) J Nanosci Nanotech 4:326
Murad E, Johnston JH (1987) In: Long GJ (ed) Mössbauer spectroscopy applied to inorganic chemistry, vol 2. Plenum Press, New York, p 507
Coquay P, Vandenberghe RE, De Grave E, Fonseca A, Piedigrosso P, Nagy JB (2002) J Appl Phys 92:1286
Gangas NH, Simopoulos A, Kostikas A, Yassoglou NJ, Filippakis S (1973) Clays Clay Minerals 21:151
Rozenson I, Heller-Kallai L (1976) Clays Clay Minerals 24:271
Reis AS Jr, Ardisson JD (2003) Clays Clay Minerals 51:33
Coey JMD (1984) In: Long GJ (ed) Mössbauer spectroscopy applied to inorganic chemistry, vol 1. Plenum Press, New York, p 443
Badreddine R, Grandjean F, Vandormael D, Fransolet AM, Long GJ (2000) Clay Minerals 35:653
Methasiri T, Yoodee K, Tang IM (1980) Physica B 101:243
Tessonnier JP, Winé G, Estournès C, Leuvrey C, Ledoux MJ, Pham-Huu C (2005) Catal Today 102–103:29
Schuele WJ, Shtrikman S, Treves D (1965) J Appl Phys 36:1010
Skoutelas AP, Karakassides MA, Petridis D (1999) Chem Mater 11:2754
Bakandritsos A, Simopoulos A, Petridis D (2005) Chem Mater 17:3468
Pérez-Cabero M, Taboada JB, Guerrero-Ruiz A, Overweg AR, Rodriguez-Ramos I (2006) Phys Chem Chem Phys 8:1230
Johnson CE, Ridout MS, Cranshaw TE, Madsen PE (1961) Phys Rev Lett 6:450
Mancier V, Delplancke JL, Delwiche J, Hubin-Franskin MJ, Piquer C, Rebbouh L, Grandjean F (2004) J Magn Magn Mater 281:27
Delplancke JL, Dille J, Reisse J, Long GJ, Mohan A, Grandjean F (2000) Chem Mater 12:946
Dong XL, Zhang ZD, Jin SR, Kim BK (2000) J Magn Magn Mater 210:143
Da Silva Borchardt F (2004) Master’s thesis, Univ Pittsburg, etd.library.pitt.edu/ETD/available/etd-10132004-194232
Kanetsuk Y, Ibaraki N, Ashida S (1991) ISIJ Int 31:304
Ruskov T, Asenov S, Spirov I, Garcia C, Mönch I, Graff A, Kozhuharova R, Leonhardt A, Mühl T, Ritschel M, Schneider C, Groudeva-Zotova S (2004) J Appl Phys 96:7514
Tokiro H, Fujii S, Muto S, Nasu S (2006) J Appl Phys 99:08Q512
Acknowledgements
The authors thank Ms. Nathalie Fagel, Dr. Leïla Rebbouh, and Prof. André Rulmont for their help during the course of this work and the referee for helpful comments. The authors acknowledge the financial support of the Ministère de la Région Wallone for grant number RW/115012 and the BINANOCO project, and of the Fonds National de la Recherche Scientifique, Belgium for grant 1.5.064.05.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Destrée, A., Long, G.J., Vatovez, B. et al. Synthesis and characterization of carbon nanotubes grown on montmorillonite clay catalysts. J Mater Sci 42, 8671–8689 (2007). https://doi.org/10.1007/s10853-007-1808-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1808-2