Abstract
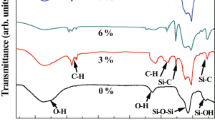
Experimental results obtained on the preparation of hydrophobic silica aerogels by ambient pressure drying method using the sodium silicate precursor with the variation of solvent exchanging process, are reported. The silica hydrogel was prepared by passing the 1.12 specific gravity sodium silicate through the Amberlite (TM) 120 Na+ resin and addition of 1 M ammonium hydroxide to silicic acid. The gel was kept in an oven for 3 h to strengthen the gel. Solvent exchange was carried out with ethanol and hexane for 36 h each followed by 24 h silylation using 20% hexamethyldisilazane (HMDZ) in hexane. Unreacted HMDZ was washed with hexane by keeping the gel in hexane for 24 h. Solvent was decanted and the gel was dried for 24 h by keeping the gel at 50 °C for 6 h, at 150 °C for 12 h and at 200 °C for 6 h. The low density (0.06 g/cm3), highly porous (96.9%), highly hydrophobic (contact angle of 160°), low thermal conductivity (0.07 W/m K) aerogels were obtained for the process of three times exchange with ethanol and three times exchange with hexane in 36 h each, followed by silylation with 20% HMDZ in hexane and two times washing with hexane in 24 h. FTIR studies showed the increase in the intensity of the Si–H and C–H bands of the aerogels with the increase of solvent exchanging times because of increase in silylation for more times of solvent exchange processes. It was found from the TG–DTA studies that the hydrophobicity of the aerogels retained up to the temperature of 325 °C. Water absorption studies show that the aerogels were remained hydrophobic up to 4 months when the aerogels were placed over the water as well as for up to 60 h in a 90% humid atmosphere. SEMs of the aerogels reveal that the pore sizes of the silica network increased, so the percentage of optical transparency decreased with the increase in exchange times with ethanol and hexane.






Similar content being viewed by others
References
Fricke J, Emmerling A (1992) In: Reisfield R, Jorgensen CK (eds) Chemistry, spectroscopy and applications of sol-gel glasses, Springer series, Structure and bonding, vol 77. Springer, Berlin, p 371
Mulder CAM, Van Lierop JG (1986) In: Fricke J (ed) Aerogels. Springer, Berlin, p 68
Attia YA (1994) Mater Technol 9:1
Hrubesh LW (1998) J Non-Cryst Solids 225:335
Carlson PJ, Johansson KE, Norrloy JK, Pingot O, Tavernier S, Van Den Bogert F, Van Luncker L (1979) Nucl Instrum Methods 160:407
Buzykaev AR, Danilyuk AF, Ganzbur SK,Gorodtskaya TA, Kolachev GM, Kravchenko EA, Mikerov VI, Minakov GD, Onuchin AP, Shamov AG, Tayursky VA (1998) J Non-Cryst Solids 225:381
Sumiyoshi T, Adachi I, Enamoto R, Iijima T, Suda R, Yokoyama M, Yokogava H (1998) J Non-Cryst Solids 225:369
Tilloston TM, Hrubesh LW, Simpsom RL, Lee RS, Swansiger RW, Simpson LR (1998) J Non-Cryst Solids 225:358
Kim K, Jang KY, Upadhye RS (1991) J Am Ceram Soc 78:1987
Pajonk GM, Teichner SJ (1985) In: Fricke J (ed) Proceedings of the first international symposium on aerogels, Wurzburg, Germany, p 193
Pajonk GM (1991) Appl Catal 72:217
Prakash SS, Brinker CJ, Hurd AJ, Rao SM (1995) Nature 374:439
Hurd AJ (1995) J Non-Cryst Solids 190:264
Yang HS, Choi SY, Hyun SH, Park HH, Hong JK (1997) J Non-Cryst Solids 221:151
Haereid S, Dahle M, Lima S, Einarsrud MA (1995) J Non-Cryst Solids 186:96
Haereid S, Nilsen E, Einarsrud MA (1996) J Porous Mater 2:315
Smith DM, Stein D, Anderson JM, Ackerman W (1995) J Non-Cryst Solids 186:104
Deshpande R, Smith DM, Brinker CJ (1992) US patent, Applic SNPCT/US 94, 05105
Lee CJ, Kim GS, Hyun SH (2002) J Mater Sci 37:2237
Venkateswara Rao A, Parvathy Rao A, Kulkarni MM (2004) J Non-Cryst Solids 350:224
Parvathy Rao A, Venkateswara Rao A, Pajonk GM (2005) J Sol-Gel Sci Technol 36(3):285
Parvathy Rao A, Pajonk GM, Venkateswara Rao A (2005) J Mater Sci 40:3481
Buzeyskaev AK, Damilyuk AF, Ganzbur SF, Kravchenko EA, Onuchin AP (1999) Nucl Instrum Methods Phys Res A 433:396
Bekerman JJ (1958) Surface chemistry, theory and applications, 2nd edn. Academic Press Inc., New York, p 343
Brinker CJ, Scherer GW (1990) Sol-gel science. Academic Press, San-Diego, p 536
Yokogawa H, Yokoyama M (1995) J Non-Cryst Solids 186:23
Venkateswara Rao A, Nilsen E, Einarsrud MA (2001) J Non-Cryst Solids 296:165
Venkateswara Rao A, Pajonk GM, Parvathy NN, Elaloui E (1994) In: Attia YA (ed) Sol-gel processing and applications. Plenum Publications, New York, p 237
Yoldas BE (1984) J Non-Cryst Solids 63:145
Hering N, Schriber K, Reidel R, Lichtenberger O, Woltersodorf J (2001) Appl Organomater Chem 15:879
Acknowledgements
The authors are highly thankful to the Department of Science and Technology (DST), New Delhi, for funding this work under the project No. SP/S2.CMP-01/2002. A. Parvathy Rao and Poonam M. Shewale are thankful for providing the fellowships in the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parvathy Rao, A., Venkateswara Rao, A., Pajonk, G.M. et al. Effect of solvent exchanging process on the preparation of the hydrophobic silica aerogels by ambient pressure drying method using sodium silicate precursor. J Mater Sci 42, 8418–8425 (2007). https://doi.org/10.1007/s10853-007-1788-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1788-2