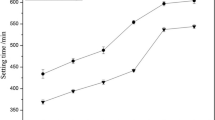
Abstract
Ordinary Portland cement (OPC) paste specimens and compacted C–S–H (I) powders were immersed in distilled water and aqueous salt solutions of varying concentration to study their volume change behavior. Immersion resulted in ionic interaction leading to various degrees of expansion, leaching, softening and dissolution of the test samples. In all cases relatively rapid expansion occurred. The expansion of C–S–H (I) in aggressive media was found to be large and fast in MgCl2, MgSO4, LiOH, LiNO3 and calcium (magnesium) acetate (CaMgAc) solutions. LiCl, CaCl2 and NaCl were moderately aggressive towards C–S–H (I) depending on the solution concentration. Trends in the length change behavior of OPC paste are similar to that of synthesized C–S–H (I). Similarities were observed between the length change behavior of compacted C–S–H (I) and the swelling of smectite clays in contact with these osmotic media. The similarities are compatible with explanations of expansion provided by both the osmotic and the electrical double layer (EDL) theories. The relationship between the expansive behavior of C–S–H and both Na and Ca Montmorillonite in contact with aqueous salt solutions is discussed extensively in the context of its significance in cement science.










Similar content being viewed by others
References
Brown PW, Taylor HFW (2000) In: Marchand J, Skalny JP (eds) Materials science of concrete: special volume on sulfate attack mechanisms. American Ceramic Society, p 73
Marchand J, Samson E, Maltais Y (2000) In: Marchand J, Skalny JP (eds) Materials science of concrete: special volume on sulfate attack mechanisms. American Ceramic Society, p 211
Litvan GG (1980) In: Proceedings of the 7th international congress on chemistry of cement, Paris, France, vol 3, pp VII-46–VII-46–VII-50
Santhanam M, Cohen MD, Olek J (2003) Cem Concr Res 33(3):325
Santhanam M, Cohen MD, Olek J (2003) Cem Concr Res 33(3):341
Taylor HFW, Famy C, Scrivener K (2001) Cem Concr Res 31:683
Skalny J, Marchand J, Odler I (2002) Sulfate attack on concrete. Spon Press, New York, p. 217
Beaudoin JJ, Raki L, Marchand J (2003) J Mater Sci 38:4957. DOI: 10.1023/B:JMSC.0000004419.94744.4F
Roßler C, Stark J, Steiniger F, Tichelaar W (2006) J Am Ceram Soc 89:627
Richardson GI (2004) Cem Concr Res 34(9):1733
Beaudoin JJ (2004) In: Ramchandran VS, Beaudoin JJ (eds) Handbook of analytical techniques in concrete science and technology, chap. II. William Andrew Publishers, New York, p 964
Greenberg SA (1954) J Phys Chem 58:362
Pointeau I, Piriou B, Fedoroff M, Bartes GM, Marmier N, Fromage F (2001) J Coll Int Sci 236:252
Taylor HFW (1986) J Am Ceram Soc 69(6):464
Terisse VH, Nonat A, Petit CJ (2001) J Coll Int Sci 244:58
Beaudoin JJ (1999) Concr Int 21(8):86
Hong S-Y, Glasser FP (1999) Cem Concr Res 29:1893
Hong S-Y, Glasser FP (2002) Cem Concr Res 32:1101
Van Olphen H (1977) An introduction to clay colloid chemistry, 2nd edn. Wiley, p 318
Mcbride MB (1994) Environmental chemistry of soils. Oxford University Press, p 416
Velde B (1992) Introduction to clay minerals: chemistry, origins, uses and environmental significances, 1st edn. Chapman and Hall, p 195
Newman ACD (1987) Chemistry of clays and clay minerals. Monograph No. 6, Mineralogical Society, p. 480
Luckham PF, Rossi S (1999) Adv Coll Int Sci 248:231
Lubertkin DS, Midlleton RS, Ottewill RH (1984) Phil Trans R Soc Lond A311:133
Huerta M, Mcquarrie DA (1991) Electrochem Acta 36(11/12):1751
Quirk JP (1952) PhD. Thesis, University of London, p 312
Norish K (1954) Discuss Faraday Soc 18:120
Aylmore LA, Quirk JP (1962) Clays Clay Miner 9:104
Kjellander R, Marcelja S, Quirk JP (1988) J Coll Int Sci 126(1):194
Catinaud S, Beaudoin JJ, Marchand J (2001) Concr Sci Eng 3:100
Feldman RF, Ramachandran VS (1989) Cemento 86(2):87
Alizadeh R, Beaudoin JJ, Raki L (2007) J Am Ceram Soc 90(2):670
Beaudoin JJ, Catinaud S, Marchand J (2001) Cem Concr Res 31:149
Shaw DJ (1992) Colloid and surface chemistry, 4th edn. Butterworth-Heinemann Ltd., p 320
Viallis H, Nonat A, Petit J-C (2001) J Coll Int Sci 244:58
Kjellander R, Marcelja S, Quirk JP (1988) J Phys Chem 92:6489
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dramé, H., Beaudoin, J.J. & Raki, L. A comparative study of the volume stability of C–S–H (I) and Portland cement paste in aqueous salt solutions. J Mater Sci 42, 6837–6846 (2007). https://doi.org/10.1007/s10853-006-1328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1328-5