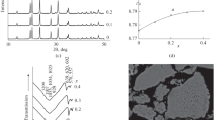
Sodium zirconium phosphate NaZr2P3O12 (hereafter NZP) crystallizes in rhombohedral (hexagonal) symmetry with the space group R-3c. The NZP-related phase of synthetic iron substituted NZP has been prepared by partial substitution on zirconium site by Fe(III). The material has been synthesized by sintering the finely powdered oxide mixture in a muffle furnace at 1,050 °C. The polycrystalline phase of Na1.2Zr1.8Fe0.2(PO4)3 has been characterized by its typical powder diffraction pattern. The powder diffraction data of 3,000 points have been subjected to general structural analysis system (GSAS) software to arrive at a satisfactory structural fit with R p = 0.0623 and R wp = 0.0915. The following unit cell parameters have been calculated: a = b = 8.83498(18) Å, c = 22.7821(8) Å and α = β = 90.0° γ = 120.0°. The structure of NZP consists of ZrO6 octahedra and PO4 tetrahedra linked by the corners to form a three-dimensional network. Each phosphate group is on a two-fold rotation axis and is linked to four ZrO6 octahedra. Each zirconium octahedron lies on a threefold rotation axis and is connected to six PO4 tetrahedra. AC conductivity of the solid solution has been measured between 303 and 773 K. The material exhibits temperature-dependent enhancement of ionic conduction by ≈400 times at elevated temperatures. The activation energies show significant change in slope at 1,000/T = 2.23(448 K).






Similar content being viewed by others
References
Scheetz BE, Agrawal DK, Brevel E, Roy R (1994) Sodium zirconium phosphate (NZP) as a host structure for Nuclear waste immobilization: a review. Waste Management 14(6):489
Breval E, Agrawal DK (1995) Brit Ceram Trans 94:27
Orlova AI, Pet’kov VI, Skiba OV (1997) In: International conference on future nuclear system, Global ‘97, Yokohama Proceedings, vol 2. p 1253
Hazen RM, Prewitt CT (1977) Am Mineral 62:309
Govindan Kutty KV, Asuvathraman R, Sridhran R (1998) J Mat Sci 33:4007
Kohler J, Imanaka N, Adachi G (1999) Chem Mater 10:1767
Verissimo Carla MS, Garrido Francisco L, Oswaldo A, Paloma C, Ana M-J, Iglesias Juan E, Rojo Jose M (1997) Solid State Ionics 100:127
Roy R, Agrawal DK, Alammo J, Roy RA (1984) Mat Res Bull 19:471
Hirose Y, Fukasawa T, Agrawal DK, Scheetz BE, Nageswaran R, Curtis JA, Limaye SY (1999) In: WM 1999 conference
Shannon RD (1976) Acta Crystallogr A 32:751
Goodenough JB, Hong HYP, Kafalas JA (1976) Mat Res Bull 11:203
Hagman L, Kierkegaard P (1968) Acta Chem Stand 22:1822
Hong HYP (1976) Mat Res Bull 11:173
Pet’kov VI, Orlova AI, Kazantsev GN, Samoilov SG, Spiridonova ML (2001) J Therm Anal Calorimetr 66:623
Buvaneswaria G, Govindan Kutty KV, Varadaraju UV (2004) Mat Res Bull 39:475
JCPDS Powder diffraction data file no. 71-0959 (2000) Compiled by International Center for Diffraction Data USA
Larson AC, Von Dreele RB, General structure analysis system technical manual, LANSCE, MS-H805, Los Alamos National Laboratory, USA
Furberg S (1955) Acta Chem Scand 9:1557
Cruickshank DWJ (1964) Acta Cryst 17:671
Nord AG, Kiekegaard P (1968) Acta Chem Scand 22:1465
West AR (1998) Solid state chemistry and its application, John Willey, New York, pp 484–487
Breval E, McKinstry HA, Agrawal DK (1994) Br Ceram Trans 93(6):239
Nowick AS, LeeWK (1989) In: Laksar AL, Chandra S (eds) Superionic solids and solid electrolytes, recent trends. Academic press, Boston pp 381–405
Le Meins JM, Bohnke O, Gourbion G (1998) Solid State Ionics 111:67
Tilement O, Angenaut J, Couturier JC, Quarton M (1991) Solid State Ionics 44:299
Acknowledgements
We gratefully acknowledge the help of Dr Ranveer Kumar Department of Physics, Dr. H.S. Gour University for electrical measurements on the samples. The authors thank the University Grant Commission, New Delhi for funding the major project no. F-12-137/2001(SR-1) and to DST, New Delhi for providing X-ray facility at Jammu University.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 2
Rights and permissions
About this article
Cite this article
Shrivastava, O.P., Kumar, N. & Chourasia, R. Synthesis, crystallographic characterization and ionic conductivity of iron substituted sodium zirconium phosphate Na1.2Zr1.8Fe0.2(PO4)3 . J Mater Sci 42, 2551–2556 (2007). https://doi.org/10.1007/s10853-006-1230-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1230-1