Abstract
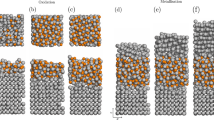
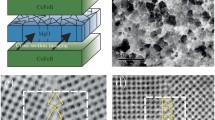
The functional properties of magnetic tunnel junctions are critically dependant on the nanoscale morphology of the insulating barrier (usually only a few atomic layers thick) that separates the two ferromagnetic layers. Three-dimensional atom probe analysis has been used to study the chemistry of a magnetic tunnel junction structure comprising an aluminium oxide barrier formed by in situ oxidation, both in the under-oxidised and fully oxidised states and before and after annealing. Low oxidation times result in discrete oxide islands. Further oxidation leads to a more continuous, but still non-stoichiometric, barrier with evidence that oxidation proceeds along the top of grain boundaries in the underlying CoFe layer. Post-deposition annealing leads to an increase in the barrier area, but only in the case of the fully oxidised and annealed structure is a continuous planar layer formed, which is close to the stoichiometric Al:O ratio of 2:3. These results are surprising, in that the planar layers are usually considered unstable with respect to breaking up into separate islands. Analysis of the various driving forces suggests that the formation of a continuous layer requires a combination of factors, including the strain energy resulting from the expansion of the oxide during internal oxidation on annealing.






Similar content being viewed by others
References
Barthélémy A, Fert A, Contour J-P, Bowen M, Cros V, de Teresa JM, Hamzic A, Faini JC, George JM, Grollier J, Montaigne F, Pailloux F, Petroff F, Vouille C (2002) J Mag Mag Mat 242–245:68
Jullière M (1975) Phys Lett 54A:225
Moodera JS, Kinder LR, Wong TM, Meservey R (1995) Phys Rev Lett 74:3273
Moodera JS, Kinder LR, Nowak J, Leclair P, Meservey R (1996) Appl Phys Lett 69:708
Moodera JS, Kinder LR (1996) J Appl Phys 79:4724
Park BG, Lee TD, Lee TH, Kim CG, Kim CO (2003) J Appl Phys 93:6423
Bae JS, Shin KH, Lee HM (2002) J Appl Phys 91:7947
Tsymbal EY, Mryasov ON, Le Clair PR (2003) J Phys Condens Matt 15:R109
Tsymbal EY, Pettifor DG (1998) Phys Rev B 58:432
Da Costa V, Tiusan C, Dimopoulos T, Ounadjela K (2000) Phys Rev Lett 85:876
Rabson DA, Jönsson-Åkerman BJ, Romero AH, Escudero R, Leighton C, Kim S, Schuller IK (2001) J Appl Phys 89:2786
Shang P, Petford-Long AK, Nickel JH, Sharma M, Anthony TC (2001) J Appl Phys 89:6874
Ozkaya D, Mcbride W, Cockayne DJH (2004) Interface Sci 12:321
Ozkaya D, Dunin-Borkowski RE, Petford-Long AK, Wong PK, Blamire MG (2000) J Appl Phys 87:5200
Stobiecki T, Kanak J, Wrona J, Czapkiewicz M, Kim CG, Kim CO, Tsunoda M, Takahashi M (2004) Phys Stat Sol A 201:1621
Zhu W, Hirschmugl CJ, Laine AD, Sinkovic B, Parkin SSP (2001) Appl Phys Lett 78:3103
Miller MK, Cerezo A, Hetherington MG, Smith GDW (1996) Atom probe field ion microscopy. Oxford University Press, Oxford
Cerezo A, Larson DJ, Smith GDW (2001) MRS Bull 26:102
Petford-Long AK, Ma YQ, Cerezo A, Larson DJ, Singleton EW, Carr BW (2005) J Appl Phys 98:124904
Larson DJ, Wissman BD, Martens RL, Viellieux RJ, Kelly TF, Gribb TT, Erskine HF, Tabat N (2001) Micro Microanal 7:24
Larson DJ, Petford-Long AK, Ma YQ, Cerezo A (2004) Acta Mater 52:2847
Cerezo A, Godfrey TJ, Sibrandij SJ, Smith GDW, Warren PJ (1998) Rev Sci Instrum 69:49
Lehnert T, Billon D, Grassl C, Grundlach KH (1992) J Appl Phys 72:3165
Zhou XW, Wadley HNG (2005) Phys Rev B 71:054418
Zhou XW, Wadley HNG, private communication
Larson DJ, Cerezo A, Clifton PH, Petford-Long AK, Martens RL, Kelly TF, Tabat N (2001) J Appl Phys 89:7517
Gas P, Bergman C, Lábár JL, Barna PB, D’heurle FM (2004) Appl Phys Lett 84:2421
Buchanan JDR, Hase TPA, Tanner BK, Chen PJ, Gan L, Powell CJ, Egelhoff WF Jr (2003) J Appl Phys 93:8044
Raynor GV, Rivlin VG (1988) Phase equilibria in iron ternary alloys. Institute of Materials, London, p 76
Macallister AJ (1990) In: Massalski TB, Okamoto H, Subramaniam PR, Kacprzak L (eds) Binary alloy phase diagrams, 2nd edn. ASM International, OH, p 136
Levin EM, Mcmurdie HF (1975) Phase diagrams for ceramicists, 1975 supplement. American Ceramic Society, Columbus, OH, pp 236
Murr LE (1975) Interfacial phenomena in metals and alloys. Addison-Wesley, London
Mchale JM, Auroux A, Perotta AJ, Navrotsky A (1997) Science 277:788
Levi G, Kaplan WD (1997) Acta Mater 51:788
Ozkaya D, Mcbride W, Cockayne DJH (2004) Interface Sci 12:321
Fei GT, Barnes JP, Petford-Long AK, Doole RC, Serna R, Gonzalo J (2002) J Phys D Appl Phys 35:916
Weast RC (1983) CRC handbook of chemistry and physics, 63rd edn. CRC Press, Boca Raton, FL
Zhou XW, Wadley HNG, Wang DX, submitted to Comput Mater Sci
Nabarro FRN (1940) Proc Roy Soc A 175:519
Christian JW (2002) The theory of transformations in metals and alloys, Part I. 2nd edn. Elsevier, pp 464
Acknowledgements
The authors are grateful to Prof. G.D.W. Smith FRS for provision of laboratory facilities and for helpful discussions during the preparation of this paper. We would also like to thank Xiaowang Zhou, University of Virginia, for valuable contributions on the mechanisms of oxide growth. Laser-pulsed 3DAP experiments were performed at Oxford nanoScience Limited, Milton Keynes, UK and we are grateful to Dr. Peter Clifton for his assistance in the collection of the data. This work was supported by funding from the Engineering and Physical Sciences Research Council. AC is also grateful for support from Oxford nanoScience Limited during the writing of this paper. Argonne National Laboratory is supported by the U.S. Department of Energy, Basic Energy Sciences – Materials Sciences, under contract (W-31-109-ENG-38.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cerezo, A., Petford-Long, A.K., Larson, D.J. et al. The formation mechanism of aluminium oxide tunnel barriers. J Mater Sci 41, 7843–7852 (2006). https://doi.org/10.1007/s10853-006-0562-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0562-1