Abstract
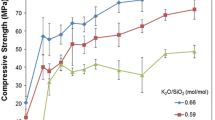
This article is the second in a two-part series and discusses inorganic polymers derived from fly ash. Part 1 [1] concerns inorganic polymers derived from a metakaolin precursor. For this study, 15 fly ash-derived inorganic polymers were produced with various compositions. The effect of the concentration of each of the four component oxides (Na2O, SiO2, Al2O3 and H2O) and two alkali cations (Na and K) on the microstructure and compressive strengths were assessed. Similar to metakaolin-derived inorganic polymers, it was observed that high-strength fly ash inorganic polymers were related to low porosity and a dense, fine-grained microstructure. Such structures were characteristic of formulations with high silica mole fractions (SiO2/Al2O3 ∼ 3.9) and low water contents, as well as those with high alkali and low alumina contents. For the latter, not only was a characteristic slower strength development with increasing alkali content observed, but there was also a limit of alkali concentration (Na2O/Al2O3 ∼1) beyond which the strength deteriorated. Furthermore, SEM micrographs disclose that the fly ash precursor dissolves more readily in the sodium-based system compared to the potassium equivalent. The interrelation between microstructures of the respective formulations and their strength development are discussed. It is observed that the charge-balancing role of the alkali cations in the fly ash formulations may be dominant compared to initial alkali dissolution reaction of the aluminosilicate fly ash particles, which is partly responsible for initial strength development.
Similar content being viewed by others
References
M. STEVESON and K. SAGOE-CRENTSIL, “Relationships Between Composition, Structure and Strength in Inorganic Polymers: Part 1—Metakaolin-Derived Inorganic Polymers”, submitted to J. Mater. Sci. (2002).
A. PALOMO, M. W. GRUTZECK and M. T. BLANCO, Cem. Concr. Res. 29 (1999) 1323.
J. G. S. VAN JAARSVELD, J. S. J. VAN DEVENTER and L. LORENZEN, Met. Mater. Trans. B 29B (1998) 283.
T. SILVERSTRIM, H. ROSTAMI, B. CLARK and J. MARTIN, in Proceedings of the 19th International Conference on Cement Microscopy. (Cincinnati, USA 1997) p. 355.
J. W. PHAIR and J. S. J. VAN DEVENTER, Miner. Eng. 14 (2001) 289.
J. G. S. VAN JAARSVELD, J. S. J. VAN DEVENTER and A. SCHWARTZMAN, Miner. Eng. 12(1) (1999) 75.
J. G. S. VAN JAARSVELD and J. S. J. VAN DEVENTER, Ind. Eng. Chem. Res. 38 (1999) 3932.
CANMET, Canada, DSS Contract No. 23440-6-9-195/01SQ, “Preliminary Examination of the Potential Use of Geopolymers for Use in Mine Tailings Management”, Final Report, 1988.
cited in J. G. S. Van JAARSVELD and J. S. J. VAN DEVENTER, Cem. Concr. Res. 29 (1999) 1189.
J. W. PHAIR, J. S. J. VAN DEVENTER and J. D. SMITH, Ind. Eng. Chem. Res. 39 (2000) 2925.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steveson, M., Sagoe-Crentsil, K. Relationships between composition, structure and strength of inorganic polymers. J Mater Sci 40, 4247–4259 (2005). https://doi.org/10.1007/s10853-005-2794-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10853-005-2794-x