Abstract
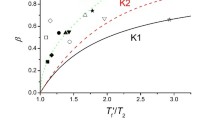
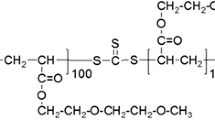
Enthalpy relaxation of polystyrene (PS) and four modified polystyrene copolymers containing co-monomers capable of forming hydrogen bonds of different strengths is described. Values of enthalpy lost (Δ H(Ta, ta)) were calculated from experimental data plotted against log (ta) and modelled using the Cowie-Ferguson (CF) semi-empirical model. This gives a set of values for three adjustable parameters, Δ H∞(Ta), log (tc) and β . Each of the parameters defines the relaxation process, which was found to be sensitive to changes in hydrogen bond strength. The introduction of hydrogen bonding causes a slower relaxation compared with PS, with a greater overall enthalpy lost measured for the all the copolymers except the styrene-co–4-hexafluoro-2-hydrox isopropyl styrene (SHFHS). Interestingly, the free volume of this copolymer measured using Positron Annihilation Lifetime Spectroscopy (PALS) was greater than that of PS. Furthermore, the SHFHS copolymer had the lowest change in heat capacity (Δ Cp) of any of the systems on passing through the glass transition, Tg. All experiments indicate that the enthalpy lost by the fully relaxed glass (Δ H∞(Ta)) is less than the theoretical amount possible on reaching the state defined by the equilibrium liquid enthalpy line (Δ Hmax(Ta)). The results are discussed with reference to the strength of interaction and free volume.
Similar content being viewed by others
References
J. M. HUTCHINSON, Prog. Polym. Sci. 20 (1995) 703.
L. C. E. STRUIK, “Physical Ageing in Polymers and Other Materials” Elsevier, Amsterdam (1978).
E. M. PEARCE, T. K. KWEI and B. Y. MIN, J. Macromol. Sci. Chem. A 21 (1984) 1181.
J. M. G. COWIE, B. G. DEVLIN and I. J. MCEWEN, Polymer 34 (1993) 4130.
J. M. G. COWIE and R. FERGUSON, ibid. 34 (1993) 2135.
J. M. G. COWIE, S. HARRIS and I. J. MCEWEN, J. Polym. Sci. Phys. Ed. 35 (1997) 1107.
A. BRUNACCI, J. M. G. COWIE, R. FERGUSON and I. J. MCEWEN, Polymer 38 (1997) 865.
N. R. CAMERON, J. M. G. COWIE, R. FERGUSON and I. MCEWAN, ibid. 41 (2000) 7255.
J. M. G. COWIE and R. FERGUSON, Macromolecules 22 (1989) 2312.
E.-A. MCGONIGLE, J. M. G. COWIE, V. ARRIGHI and R. A. PETHRICK, submitted to Polymer.
N. R. CAMERON, J. M. G. COWIE, R. FERGUSON and I. MCEWAN, Polymer 42 (2001) 6991.
J. L. GÓMEZ RIBELLES and M. MONLEÓN PRADAS, Macromolecules 28 (1995) 5867.
A. BRUNACCI, J. M. G. COWIE, R. FERGUSON, J. L. GÓMEZ RIBELLES and A. VIDAURRE GARAYO, ibid. 29 (1996) 7976.
D. M. BIGG, Polym. Engng. Sci. 36 (1996) 737.
W. O. KENYON and G. P. WAUGH, J. Polym. Sci. 32 (1958) 83.
C. CHENG and E. M. PEARCE, J. Polym. Sci. Polym. Chem. Ed. 18 (1980) 1651.
R. ARSHADY, G. W. KENNER and A. LEDWITH, J. Polym. Sci. Polym. Chem. 12 (1974) 2017.
G. LI, J. M. G. COWIE and V. ARRIGHI, J. Appl. Polym. Sci. 74 (1999) 639.
J. M. G. COWIE and A. A. N. REILLY, Polymer 33 (1992) 4814.
M. J. RICHARDSON and N. G. SAVILL, ibid. 16 (1975) 753.
R. A. PETHRICK, F. M. JACOBSEN, O. E. MOGENSEN and M. ELDRUP, J. Chem. Soc., Faraday II 76 (1980) 225.
W. J. DAVIES and R. A. PETHRICK, Eur. Polym. J. 30 (1994) 1289.
P. KIRKEGAARD, M. ELDRUP, O. E. MOGENSEN and N. PEDERSEN, Comp. Phys. Commun. 23 (1981) 307.
S. N. CASSU and M. I. FELISBERTI, Polymer 38 (1997) 3907.
G. LEVITA and L. C. E. STRUIK, ibid. 24 (1983) 1071.
E.-A. MCGONIGLE, J. M. G. COWIE, V. ARRIGHI and R. A. PETHRICK, to be submitted for publication.
C. WÁSTLUND, H. BERNSTSSON and F. H. J. MAURER, Macromolecules 31 (1998) 3322.
E.-A. MCGONIGLE, J. J. LIGGAT, R. A. PETHRICK, S. D. JENKINS, J. H. DALY and D. HAYWARD, Polymer 42 (2001) 2413.
M. K. GRAY, H. ZHOU, S. T. NGUYEN and J. M. TORKELSON, ibid. 45 (2004) 4777.
R. A. PETHRICK, Prog. Polym. Sci. 22 (1997) 1.
A. J. HILL, S. WEINHOLD, G. M. STACK and M. R. TANT, Eur. Polym. J. 32 (1996) 843.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mcgonigle, EA., Cowie, J.M.G., Arrighi, V. et al. Enthalpy relaxation and free volume changes in aged styrene copolymers containing a hydrogen bonding co-monomer. J Mater Sci 40, 1869–1881 (2005). https://doi.org/10.1007/s10853-005-1206-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10853-005-1206-6