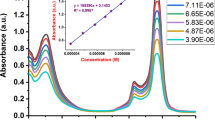
Abstract
Bis-benzyl 2-(oxy) benzoate substituted axially silicon phthalocyanine was synthesized by the reaction of silicon phthalocyanine dichloride and benzyl salicylate compounds. Characterization of the compound was done by FT-IR, 1H NMR, 13C NMR, UV-visible and Mass spectrum. Photochemical and photophysical properties of new silicon phthalocyanine (SiPc) was investigated. Biological properties of SiPc was carried out by several different parameters. The highest antioxidant ability of 73.18% was obtained at 100 mg/L concentration while the lowest antioxidant activity of 38.46% was obtained at 6.25 mg/L concentration. The antimicrobial effects of SiPc were investigated against different bacteria and microfungi. The results regarding the antimicrobial activity of this compound 3 showed that E. faecalis (ATCC 29,212) was the most sensitive microorganism to the tested compounds, while C. tropicalis was the most resistant microorganism. In addition, when the antimicrobial photodynamic treatment of SiPc was examined, a better activity was observed against all microorganisms. DNA fragmentation activity and microbial cell viability of compound 3 was investigated. SiPc showed excellent DNA nuclease activity and 99.96% inhibition of cell viability at 100 mg/L. The effect of compound 3 on antibiofilm activity fabricated by S. aureus and P. aureginosa was also measured and a good biofilm inhibition values of 86.51% and 75.24% was achieved at 50 mg/, respectively. In addition, when the antidiabetic effects of the compounds were examined, it showed an antidiabetic effect of 20.14% at 400 mg/L.












Similar content being viewed by others
References
Guowei, L., et al.: Fluorinated/non-fluorinated triphenylamine axially substituted silicon phthalocyanine: Synthesis and photophysical properties. Inorg. Chem. Comm. 141, 109490 (2022)
Yaşa Atmaca, G., et al.: Comparison of sonodynamic, photodynamic and sonophotodynamic therapy activity of fluorinated pyridine substituted silicon phthalocyanines on PC3 prostate cancer cell line. Photodiagnosis Photodyn Ther. 42, 103339 (2023)
Mendes, L.: Metallated phthalocyanines and their hydrophilic erivatives for multi-targeted oncological photodynamic therapy. J. Photochem. Photobiol. 234, 112500 (2022)
Azzouzi, S., et al.: Novel iron (III) phthalocyanine derivative functionalized semiconductor based transducers for the detection of citrate. Org. Electron. 34, 200–207 (2016)
Aziz, T.: A flexible nickel phthalocyanine resistive random access memory with multi-level data storage capability. J. Mater. Sci. Technol. 86, 151–157 (2021)
Choi, S.-H., et al.: Electro-optical characteristics of polymer-dispersed liquid crystal containing copper (II) phthalocyanine as a function of UV irradiation time. J. Mol. Liq. 363, 119821 (2022)
Ağırtaş, M.S., et al.: Synthesis and Sensor properties of Silicon Phthalocyanine axially substituted with Bis-(Prop-2-Ynyloxy) groups and polymeric phthalocyanines bearing PEG substituent by click Chemistry. Polycycl. Aromat. Compd. 43(4), 3278–3290 (2023). https://doi.org/10.1080/10406638.2022.2067195
Szostak, J., et al.: Photovoltaic properties of cadmium selenide–titanyl phthalocyanine planar heterojunction devices. Chem. Phys. 456, 57–60 (2015)
Şen, P., et al.: Synthesis and electrochemical, electrochromic and electrical properties of novel s-triazine bridged trinuclear zn(II), Cu(II) and Lu(III) and a tris double-decker Lu(III) phthalocyanines. Synth. Met. 161, 1245–1254 (2011)
Gounden, D., et al.: Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 420, 213359 (2020)
Wang, S., et al.: Graphene carbon black as catalyst support: The influences of iron phthalocyanine loading and carbon black additive amount on the power generation performance of direct glucose fuel cell. Fuel. 315, 123227 (2022)
Liu, G.: Fluorinated/non-fluorinated triphenylamine axially substituted silicon phthalocyanine: Synthesis and photophysical properties. Inorg. Chem. Commun. 141, 109490 (2022)
Güngördü Solğun, D., et al.: Synthesis of novel tetra (4-tritylphenoxy) substituted metallophthalocyanines and investigation of their aggregation, photovoltaic, solar cell properties. Inorg. Nano-Metal Chem. 48(10), 508–514 (2018)
Ağırtaş, M.S.: Highly soluble phthalocyanines with hexadeca tert-butyl substituents. Dyes Pigm. 79, 247–251 (2008)
Berezin, D.B., et al.: Aggregation of water soluble octaanionic phthalocyanines and their photoinactivation antimicrobial effect in vitro. Mendeleev Commun. 30, 621–623 (2020)
Kırbaç, E., Erdoğmuş, A.: New non-peripherally substituted zinc phthalocyanines; synthesis, and comparative photophysicochemical properties. J. Mol. Struct. 1202, 127392 (2020)
Kobayashi, H., et al.: The chemical basis of cytotoxicity of siliconphthalocyanine-based near infrared photoimmunotherapy (NIR-PIT) and its implications for treatment monitoring. Curr. Opin. Chem. Biol. 74, 102289 (2023)
Erdağ Maden, Y., et al.: Electrochemical and spectroelectrochemical characterizations of phthalocyanines bearing peripherally tetra-4-carboxyethylenephenoxy anchoring groups and usage as photosensitizers of dye-sensitized solar cell. J. Electroanal. Chem. 929, 117104 (2023)
Ağırtaş, M.S., et al.: Novel metal (II) phthalocyanines with 3,4,5-trimethoxybenzyloxy-substituents: Synthesis, characterization, aggregation behaviour and antioxidant activity. Dyes Pigm. 96, 152–157 (2013)
Barut, B., Demirbaş, U.: Synthesis, anti-cholinesterease, α-glucosidase inhibitory, antioxidant and DNA nuclease properties of non-peripheral triclosan substituted metal-free, copper(II), and nickel(II) phthalocyanines. J. Organomet. Chem. 923, 121423 (2020)
Arslan, H., et al.: Antimicrobial and antioxidant activity of phenolic extracts from walnut (Juglans regia L.) green husk by using pressure-driven membrane process. J. Food Sci. Technol. 73–83 (2023)
Farajzadeh, N., et al.: Biological properties of hexadeca-substituted metal phthalocyanines bearing different functional groups. J. Inorg. Biochem. 111888 (2022)
M’barek, I., et al.: Nanocellulose synthesis from Tamarix aphylla and preparation of hybrid nanocellulose composites membranes with investigation of antioxidant and antibacterial effects. Sep. Purif. Technol. 120815 (2022)
Ağırtaş, M.S.: Fluorescence properties in different solvents and synthesis of axially substituted silicon phthalocyanine bearing bis-4-tritylphenoxy units. Heterocycl. Comm. 26(1), 130–136 (2020). https://doi.org/10.1515/hc-2020-0113
Günsel, A., et al.: Novel potential metabolic enzymes inhibitor, photosensitizer and antibacterial agents based on water-soluble phthalocyanine bearing imidazole derivative. J. Mol. Struct. 1237, 130402 (2021)
Kayir, N., Gorduk, S.: Synthesis, characterization, and investigation photophysicochemical properties of axially 2-hydroxymethyl-1,4-benzodioxan di-substituted Silicon(IV) phthalocyanine. J. Organomet. Chem. 990, 122661 (2023)
Yasa Atmaca, G., et al.: Novel axially carborane-cage substituted silicon phthalocyanine photosensitizer; synthesis, characterization and photophysicochemical properties. Spectrochim Acta Mol. Biomol. Spectrosc. 137, 244–249 (2015)
Lakowicz, J.R.: Principles of Fluorescence Spectroscopy, 3rd edn. Center for Fluorescence Spectroscopy, University of Maryland School of Medicine, Baltimore (2006)
Celik, G., et al.: Novel axially symmetric and unsymmetric silicon (IV) phthalocyanines having anti-inflammatory groups-: Synthesis, characterization and their biological properties. Dalton Trans. 7517–7529 (2022)
Colak, S., et al.: Ynthesis, characterization, solution and antioxidant properties of novel tetrakis{4-[N-((3-dimethylamino)propyl)amide]phenoxy}nickel (II) phthalocyanine and its water soluble derivatives. J. Organomet. Chem., 83–89 (2016)
Günsel, A., et al.: Novel biologically active metallophthalocyanines as promising. J. Mol. Struct. 127127 (2020)
Öztürmen, B., et al.: Synthesis, characterization, and α-glucosidase, cholinesterases, and tyrosinase inhibitory effects of axial substituted silicon and peripheral tetra-substituted copper (II), manganese (III) phthalocyanines. Appl. Organomet. Chem. 36(8) (2022)
Değirmencioğlu, I., et al.: The synthesis of novel piperazine-benzodioxole substituted phthalocyanines and investigation of their α-amylase and tyrosinase inhibition properties. J. Organomet. Chem. 122012 (2021)
Barut, B., et al.: Non-aggregated axially disubstituted silicon phthalocyanines: Synthesis, DNA cleavage and in vitro cytotoxic/phototoxic anticancer activities against SH-SY5Y cell line. Dyes Pigm. 107794 (2020)
Farajzadeh, N., et al.: Anticancer and biological properties of new axially disubstituted silicon phthalocyanines. Dalton Trans. 19 (2022) (2022)
Masilela, N., et al.: Axial coordination of zinc and silicon phthalocyanines to silver and gold nanoparticles: An investigation of their photophysicochemical and antimicrobial behavior. J. Porphyr. Phthalocyanines 17, (2013)
Strokov, K., Galstyan, A.: Chitosan-Silicon Phthalocyanine Conjugate as Effective Photo-Functional Hydrogel for Tracking and Killing of Bacteria. Eur. J. Org. Chem. 7327332 (2020)
Zhao, Y., et al.: A novel silicon(IV) phthalocyanine-oligopeptide conjugate as a highly efficient photosensitizer for photodynamic antimicrobial therapy. Dyes Pigm. 107834 (2020)
Biyiklioglu, Z., et al.: Synthesis and antimicrobial photodynamic activities of axially {4-[(1E)-3-oxo-3-(2-thienyl)prop-1-en-1-yl]phenoxy} groups substituted silicon phthalocyanine, subphthalocyanine on Gram-positive and Gram-negative bacteria. Dyes Pigm. 149–158 (2019)
Taşkın, G., et al.: Axially paraben substituted silicon(IV) phthalocyanines towards dental pathogen Streptococcus mutans: Synthesis, photophysical, photochemical and in vitro properties. J. Photochem. Photobiol A 31–40 (2015)
Author information
Authors and Affiliations
Contributions
M. S. A. wrote the main manuscript text. D. G. S. prepared the synthesis and Figs. 1, 2, 3, 4 and 5. S. Ö. prepared text and biological properties. G. T. prepared related biological shapes. All authors reviewed the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest statements.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solğun, D.G., Özdemir, S., Ağırtaş, M.S. et al. Synthesis and biological properties of benzyl 2-(oxy)benzoate-substituted silicon phthalocyanine. J Incl Phenom Macrocycl Chem 104, 137–148 (2024). https://doi.org/10.1007/s10847-024-01226-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-024-01226-4