Abstract
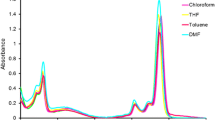
Axially bis-substituted silicon phthalocyanine was synthesized from the reaction of 2-(3,4-dimethoxy phenyl) ethanol and SiPcCl2. The structure of the compound was justified by FT-IR, 1 H NMR, 13 C NMR, UV-Vis, and mass spectra. The conglomeration action of the phthalocyanine compound was determined by UV-visible spectra at different concentrations and in different solvents. Some parameters of this axial silicon phthalocyanine compound were investigated by computational chemistry. Structural optimization of axial silicon phthalocyanine substituted with compound, HOMO-LUMO energies and MEP maps was performed by density functional theory (DFT) studies. In addition, a chemical bond analysis of the molecule was performed with quantum theory atom in molecules (QTAIM). Finally, a molecular docking study was applied to this new phthalocyanine molecule in its binding mechanism.
Graphical Abstract
Axially bis-substituted silicon phthalocyanine was synthesized by the newly designed reaction. The structure was characterized. Some parameters of this axial silicon phthalocyanine compound were investigated and analyzes were made with DFT studies and QTAIM. Finally, molecular docking work was applied.













Similar content being viewed by others
References
Çakır, V., Arslan, T.: Synthesis and biological evaluation of new silicon(IV) phthalocyanines as carbonic anhydrase and cholinesterase inhibitors. Inorganica Chim. Acta. 530, 120678 (2022)
Ağırtaş, M.S., et al.: Synthesis, characterization, and electrochemical and electrical properties of novel mono and ball-type metallophthalocyanines with four 9,9-bis(4-hydroxyphenyl)fluorene. Dalton Trans. 40, 3315–3324 (2011)
Sridevi, B.R., Hoskeri, P.A., Joseph, C.M.: Effect of annealing on the optical, structural and electrochromic properties of vacuum evaporated manganese phthalocyanine thin films. Thin Solid Films. 723, 138584 (2021)
Germinario, G., Werf, I.D., Sabbatini, L.: Pyrolysis gas chromatography mass spectrometry of two green phthalocyanine pigments and their identification in paint systems. JAAP. 115, 175–183 (2015)
Ilgün, C., et al.: Novel Co and Zn-Phthalocyanine dyes with octa-carboxylic acid substituents for DSSCs. Sol. Energy. 218, 169–179 (2021)
Zhou, J., et al.: Self-assembly and ionic conductivity of phthalocyanine-containing liquid-crystalline compound films. Thin Solid Films. 709, 138148 (2020)
Ağırtaş, M.S.: Synthesis and characterization of novel symmetrical phthalocyanines substituted with four benzo [d] [1,3] dioxol-5-ylmethoxy groups. Inorganica Chim. Acta. 360, 2499–2502 (2007)
Chen, L., et al.: Cobalt phthalocyanine as an efficient catalyst for hydrogen evolution reaction. Int. J. Hydrog Energy. 46, 19338–19346 (2021)
Türkan, F., et al.: Determination of anticancer properties and inhibitory effects of some metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, alpha-glycosidase of some compounds with molecular docking study. J. Biomol. Struct. Dyn. 39, 3693–3702 (2021)
Ağırtaş, M.S.: Fluorescence properties in different solvents and synthesis of axially substituted silicon phthalocyanine bearing bis-4-tritylphenoxy units. Heterocycl. Comm. 26, 130–136 (2020)
Sindelo, A., Mafukidze, D.M., Nyokong, T.: Fabrication of asymmetrical morpholine phthalocyanines conjugated chitosan-polyacrylonitrile nanofibers for improved photodynamic antimicrobial chemotherapy activity. Photodiagnosis Photody Ther. 38, 102760 (2022)
Tang, F., et al.: Carboxymethyl chitosan-zinc(II) phthalocyanine conjugates: Synthesis, characterization and photodynamic antifungal therapy. Carbohydr. Polym. 235, 115949 (2020)
Zhao, Y., et al.: A novel silicon(IV) phthalocyanine-oligopeptide conjugate as a highly efficient photosensitizer for photodynamic antimicrobial therapy. Dyes Pigm. 172, 107834 (2020)
Zheng, B.-D., et al.: Recent advances in supramolecular activatable phthalocyanine-based photosensitizers for anti-cancer therapy. Coord. Chem. Rev. 447, 214155 (2021)
Salih Ağırtaş, M.: Highly soluble phthalocyanines with hexadeca tert-butyl substituents. Dyes Pigm. 79, 247–251 (2008)
Openda, Y.I., Babu, B., Nyokong, T.: Novel cationic-chalcone phthalocyanines for photodynamic therapy eradication of S. aureus and E. coli bacterial biofilms and MCF-7 breast cancer. Photodiagnosis Photody Ther. 38, 102863 (2022)
Ezquerra Riega, S.D., et al.: Chalcogen bearing tetrasubstituted zinc (II) phthalocyanines for CT26 colon carcinoma cells photodynamic therapy. Dyes Pigm. 201, 110110 (2022)
Lamch, Å., et al.: Folate-directed zinc (II) phthalocyanine loaded polymeric micelles engineered to generate reactive oxygen species for efficacious photodynamic therapy of cancer. Photodiagnosis Photody Ther. 25, 480–491 (2019)
Matshitse, R., et al.: Photodynamic therapy characteristics of phthalocyanines in the presence of boron doped detonation nanodiamonds: Effect of symmetry and charge. Photodiagnosis Photody Ther. 37, 102705 (2022)
Smith, M.R., et al.: A guardian residue hinders insertion of a Fapy•dGTP analog by modulating the open-closed DNA polymerase transition. Nucleic Acids Res. 47, 3197–3207 (2019)
Tumbale, P., et al.: Aprataxin resolves adenylated RNA–DNA junctions to maintain genome integrity. Nature. 506, 111–115 (2014)
Khnifira, M., et al.: A combined molecular dynamic simulation, DFT calculations, and experimental study of the eriochrome black T dye adsorption onto chitosan in aqueous solutions. Int. J. Biol. Macromol. 166, 707–721 (2021)
Kaya, S., Kaya, C.: A new equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 1052, 42–46 (2015)
Grabowski, S.J., Ugalde, J.M.: Bond Paths Show Preferable Interactions: Ab Initio and QTAIM Studies on the X – H···π Hydrogen Bond. J. Phys. Chem. A. 114, 7223–7229 (2010)
Huang, Q.-R., Kingham, J.R., Kaltsoyannis, N.: The strength of actinide–element bonds from the quantum theory of atoms-in-molecules. Dalton Trans. 44, 2554–2566 (2015)
Abdolmaleki, A., Eskandari, K., Molavian, M.R.: Sulfonated or phosphonated membranes? DFT investigation of proton exchange in poly(oxadiazole) membranes. Polymer. 87, 181–193 (2016)
Sangeetha, K., et al.: The study of inter and intramolecular hydrogen bonds of NLO crystal melaminium hydrogen malonate using DFT simulation, AIM analysis and Hirshfeld surface analysis. Mater. Today: Proc. 25, 307–315 (2020)
Frisch, M.J., et al.: Gaussian 16 Rev. C.01. : Wallingford, CT. (2016)
Bourass, M., et al.: DFT and TD-DFT calculation of new thienopyrazine-based small molecules for organic solar cells. Chem. Cent. J. 10, 67 (2016)
Chen, K., et al.: Unusual odd-electron fragments from even-electron protonated prodiginine precursors using positive-ion electrospray tandem mass spectrometry. J. Am. Soc. Mass. Spectrom. 19, 1856–1866 (2008)
Mumit, M.A., et al.: DFT studies on vibrational and electronic spectra, HOMO–LUMO, MEP, HOMA, NBO and molecular docking analysis of benzyl-3-N-(2, 4, 5-trimethoxyphenylmethylene) hydrazinecarbodithioate. J. Mol. Struct. 1220, 128715 (2020)
Pendás, Ã.M., Gatti, C.: 3 Quantum theory of atoms in molecules and the AIMAll software.J.C.B.A.43(2021)
Bensouilah, N., et al.: Host-guest complex of N-(2-chloroethyl), N-nitroso, N′, N′-dicyclohexylsulfamid with β-cyclodextrin: Fluorescence, QTAIM analysis and structure-chemical reactivity. J. Mol. Struct. 1146, 179–190 (2017)
Yildiko, Ã., et al.: Synthesis, enzymes inhibitory properties and characterization of 2- (bis (4-aminophenyl) methyl) butan-1-ol compound: Quantum simulations, and in-silico molecular docking studies. J. Indian Chem. Soc. 98, 100206 (2021)
TEKEŞ, A.T., et al.: Insilico Molecular Docking Studies of THBF Compound: TD-DFT Simulations and Drug Design. JIST. 11, 2955–2966 (2021)
; Schrödinger Release 2017-3: Schrödinger Suite 2017-3 Protein Preparation Wizard, Epik, Schrödinger, L.L.C., New York, N.Y., Impact, Schrödinger, L.L.C., New York, N.Y.: 2017; LigPrep, Schrödinger, LLC, New York, NY, 2017; Prime, Schrödinger, LLC, New York, NY, 2017; QikProp,Schrödinger, LLC, New York, NY, 2017. (2017)
Wang, G., et al.: Design, synthesis, biological evaluation and molecular docking studies of new chalcone derivatives containing diaryl ether moiety as potential anticancer agents and tubulin polymerization inhibitors. Bioorg. Chem. 95, 103565 (2020)
BIOVIA Discovery Studio D. SYSTÈMES:. BIOVIA Corporate Europe, BIOVIA 334 Cambridge Science Park, Cambridge CB4 0WN, England. (2016). http://accelrys.com/products/collaborative-science/biovia-discovery-studio/
Rad, A.S., et al.: DFT calculations towards the geometry optimization, electronic structure, infrared spectroscopy and UV–vis analyses of Favipiravir adsorption on the first-row transition metals doped fullerenes; a new strategy for COVID-19 therapy. Spectrochim. Acta - A: Mol. Biomol. Spectrosc. 247, 119082 (2021)
Karakas, A., et al.: Theoretical Diagnostics of Second and Third-order Hyperpolarizabilities of Several Acid Derivatives %. Open. Chem. J. 17, 151–156 (2019)
Rahuman, M.H., et al.: Investigations on 2-(4-Cyanophenylamino) acetic acid by FT-IR,FT-Raman, NMR and UV-Vis spectroscopy, DFT (NBO, HOMO-LUMO, MEP and Fukui function) and molecular docking studies.Heliyon6, (2020)
Tosso, R.D., et al.: The electronic density obtained from a QTAIM analysis used as molecular descriptor. A study performed in a new series of DHFR inhibitors. J. Mol. Struct. 1134, 464–474 (2017)
Fuster, F., Grabowski, S.J.: Intramolecular Hydrogen Bonds: the QTAIM and ELF Characteristics. T J. Phys. Chem. A. 115, 10078–10086 (2011)
Yildiko, U., Tanriverdi, A.A.: A novel sulfonated aromatic polyimide synthesis and characterization: Energy calculations, QTAIM simulation study of the hydrated structure of one unit. BKCS. 43, 822 (2022)
Jenkins, S., Morrison, I.: The chemical character of the intermolecular bonds of seven phases of ice as revealed by ab initio calculation of electron densities. Chem. Phys. Lett. 317, 97–102 (2000)
Yildiko, U., Tanriverdi, A.A.: Synthesis and characterization of pyromellitic dianhydride based sulfonated polyimide: Survey of structure properties with DFT and QTAIM. J. Polym. Res. 29, 1–17 (2022)
Bianchi, R., et al.: Multipole-refined charge density study of diopside at ambient conditions. Phys. Chem. Miner. 32, 638–645 (2005)
Kamaal, S., et al.: A new copper(II)-based layered coordination polymer: Crystal structure, topology, QTAIM analysis, experimental and theoretical magnetic properties based on DFT combined with broken-symmetry formalism (BS-DFT). Polyhedron. 193, 114881 (2021)
Zhao, D.-X., Yang, Z.-Z.: Theoretical Exploration of the Potential and Force Acting on One Electron within a Molecule. J. Phys. Chem. A. 118, 9045–9057 (2014)
Zhou, H.-X., Pang, X.: Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chem. Rev. 118, 1691–1741 (2018)
Grishina, A.M., Potemkin, A.V.: Topological Analysis of Electron Density in Large Biomolecular Systems. Curr. Drug Discov Technol. 16, 437–448 (2019)
Bader, R.F.W., Anderson, S.G., Duke, A.J.: Quantum topology of molecular charge distributions. 1. J. Am. Chem. Soc. 101, 1389–1395 (1979)
ALTUN, K., et al.: Structural and spectral properties of 4-(4-(1-(4-Hydroxyphenyl)-1-phenylethyl) phenoxy) phthalonitrile: Analysis by TD-DFT method, ADME analysis and docking studies. IJCT. 5, 147–155 (2021)
Acknowledgements
We would like to thank Van Yüzüncü Yıl University Scientific Research Projects Unit and Science Application and Research Center for their support (FBA-2022-9776).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solgun, D.G., Tanriverdi, A.A., Yildiko, U. et al. Synthesis of axially silicon phthalocyanine substituted with bis- (3,4-dimethoxyphenethoxy) groups, DFT and molecular docking studies. J Incl Phenom Macrocycl Chem 102, 851–860 (2022). https://doi.org/10.1007/s10847-022-01164-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-022-01164-z