Abstract
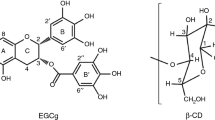
The inclusion of catechins with cyclodextrin (CD) is performed for the purpose of improving the water solubility and reducing the bitterness of catechins. In this study, the effect of catechins’ conformation on the formation of an inclusion complex between catechins and β-CD was examined by density functional theory (DFT) calculation at the B3PW91/cc-pVDZ level. It is known that the main components of catechins in tea leaves are (−)-epigallocatechin (EGC), which is a non-gallate-type catechin, and (−)-epigallocatechin gallate (EGCg), which is a gallate-type catechin. Catechins have a plurality of sites that can be included by β-CD (EGC: AC ring and B ring, EGCg: AC ring, B ring, and B′ ring). First, initial models for the calculation in which each ring of catechins gradually approaches into the cavity of β-CD were built. These initial models were optimized in water and then the optimized structure of the inclusion complex in water was determined. From the results of calculation, the degree of penetration of each ring into the β-CD cavity, the complex formation energy, and the number of intermolecular hydrogen bonds were examined. The results of calculation showed that the AC ring of catechins is most deeply included in the β-CD cavity and the B′ ring of catechins forms an energetically stable complex with β-CD. It was found that the inclusion complex of catechins and β-CD is stabilized energetically by intermolecular hydrogen bonds.










Similar content being viewed by others
References
Braicu, C., Pilecki, V., Balacescu, O., Irimie, A., Neagoe, I.B.: The relationships between biological activities and structure of flavan-3-ols. Int. J. Mol. Sci. 12, 9342–9353 (2011)
Reygaert, W.C.: Green tea catechins: their use in treating and preventing infectious diseases. Biomed. Res. Int. 2018, 1–9 (2018). https://doi.org/10.1155/2018/9105261
Muramatsu, K., Fuyuko, M., Hara, Y.: Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 32, 613–622 (1986)
Kajimoto, O., Kajimoto, Y., Yabune, M., Nozawa, A., Nagata, K., Kakuda, T.: Tea catechins reduce serum cholesterol levels in mild and borderline hypercholesterolemia patients. J. Clin. Biochem. Nutr. 33, 101–111 (2003)
Nagao, T., Hase, T., Tokimitsu, I.: A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity 15(6), 1473–1483 (2007)
Huang, S.-W., Frankel, E.N.: Antioxidant activity of tea catechins in different lipid systems. J. Agric. Food Chem. 45, 3033–3038 (1997)
Ishizu, T., Kintsu, K., Yamamoto, H.: NMR study of the solution structures of the inclusion complexes of β-cyclodextrin with (+)-catechin and (−)-epicatechin. J. Phys. Chem. B 103, 8992–8997 (1999)
Hayashi, N., Chen, R., Hiraoka, M., Ujihara, T., Ikezaki, H.: β-Cyclodextrin/surface plasmon resonance detection system for sensing bitter-astringent taste intensity of green tea catechins. J. Agric. Food Chem. 58, 8351–8356 (2010)
Kriz, Z., Koca, J., Imberty, A., Charlot, A., Auzely-Velty, R.: Investigation of the complexation of (+)-catechin by β-cyclodextrin by a combination of NMR, microcalorimetry and molecular modeling techniques. Org. Biomol. Chem. 1, 2590–2595 (2003)
Ikeda, H., Moriwaki, H., Matsubara, T., Yukawa, M., Iwase, Y., Yukawa, E., Aki, H.: Mechanism of interaction between risperidone and tea catechin (2) influence of presence of galloyl group in catechin on insoluble complex formation with risperidone. Yakugaku Zasshi 132, 145–153 (2012)
Ohata T., Ikeda H., Yukawa M., Iwase Y., Inenaga K., Aki H.: In: Abstructs of International Confederation for Thermal Analysis and Calorimetry Congress 2016, vol. 33 (2016)
Ivanov, P.M.: CONFLEX/MM3 search/minimization study of the conformations of the macrolide antibiotic tylosin. J. Mol. Struct. 606, 217–229 (2002)
Stewart, J.J.P.: Optimization of parameters for semiempirical methods II. Applications. J. Comp. Chem. 10(2), 221–264 (1989)
Mineva, T., Russo, N., Sicilia, E.: Solvation effects on reaction profiles by the polarizable continuum model coupled with the Gaussian density functional method. J. Comp. Chem. 19(3), 290–299 (1998)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: “Gaussian 09”, Revision D.01; Gaussian, Inc., USA) Gaussian, Inc., Wallingford CT (2013)
Pereira, R.A., Anconi, C.P.A., Nascimento Jr., C.S., De Almeida, W.B., Santos, H.F.: Stability and spatial arrangement of the 2,4-dichlorophenoxyacetic acid and β-cyclodextrin inclusion compound: A theoretical study. Chem. Phys. Lett. 633, 158–162 (2015)
Ignaczak, A., Orszaṅski, Ł, Adamiak, M., Olejniczak, A.B.: Comparative DFT study of inclusion complexes of thymidine-carborane conjugate with β-cyclodextrin and heptakis (2, 6-O-dimethyl)-β-cyclodextrin in water. J. Mol. Liq. 315, 113767 (2020)
Lichtenthaler, F.W., Immel, S.: Cyclodextrins, cyclomannins, and cyclogalactins with five and six (1→ 4)-linked sugar units: a comparative assessment of their conformations and hydrophobicity potential profiles1. Tetrahedron 5(11), 2045–2060 (1994)
Goto, H.: Further developments in the algorithm for generating cyclic conformers. Test with cycloheptadecane. Tetrahedron Lett. 33(10), 1343–1346 (1992)
Kamo, S., Saito, T., Kusakabe, Y., Tomoshige, S., Uchiyama, M., Tsubaki, K., Kuramochi, K.: Synthetic and biological studies of juglorubin and related naphthoquinones. J. Org. Chem. 84, 13957–13966 (2019)
Grégory, G.J., Efstathia, I., Véronique, M., Céline, B., Florence, L., Olivier, P.T., Robert, K., Vassilios, R.: Determination of the absolute configuration and evaluation of the in vitro antitumor activity of dilospirane B. Phytochem. Lett. 5, 747–751 (2012)
Acknowledgements
The computations were performed using the Research Institute for Information Technology (Kyushu University, Japan) and Research Center for Computational Science (National Institutes of Natural Sciences, Japan)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ikeda, H., Ohata, T., Yukawa, M. et al. Calculation study on complex formation of catechins with β-cyclodextrin using density function theory. J Incl Phenom Macrocycl Chem 100, 99–107 (2021). https://doi.org/10.1007/s10847-021-01057-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-021-01057-7