Abstract
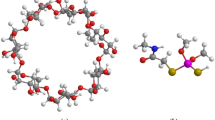
Meta-aminophenol (m-AP) was used as a guest molecule to be incorporated into the hydrophobic nano-cavity of the host molecule β-cyclodextrin (β-CD) to form host–guest inclusion complex in aqueous medium. For this, we employed spectrometric titration method, i.e., β-CD was added gradually in an aqueous solution of m-AP maintaining a fixed concentration of m-AP. We investigated the changes in the photophysical properties of m-AP through inclusion complex formation in β-CD in aqueous medium by using steady state and time-resolved spectroscopic techniques as well as density functional theory (DFT) computations. Benesi–Hildebrand method was used to find the stoichiometry and association constant at two concentration ranges of β-CD. The average fluorescence lifetime of m-AP was found to increase from 0.17 ns in absence of β-CD to 3.69 ns in presence of β-CD (highest concentration used). DFT computations carried out in water medium clearly indicate that the inclusion complex is more stable for the cis-rotameric conformation of m-AP rather than trans.








Similar content being viewed by others
References
Zhou, X., Liang, J.F.: A fluorescence spectroscopy approach for fast determination of β-cyclodextrin-guest binding constants. J. Photochem. Photobiol. A 349, 124–128 (2017)
Majhi, K., Khatun, R., Jana, S., Hajra, A., Shukla, A., Maiti, P., Dey, A., Ray, P.P., Sinha, S.: Synthesis and characterization of host–guest inclusion complex of m-cresol with β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 90, 61–73 (2018)
Prashanthi, S., Kumar, P.H., Siva, D., Bangal, P.R.: Investigation of supramolecular stoichiometry and dynamic for inclusion complex of water soluble porphyrin with cucurbit [7] uril by fluorescence correlation spectroscopy. J. Photochem. Photobiol. A 284, 27–35 (2014)
Reis, S., Liberto, N.A., Fernandes, S.A., de Fátima, A., Almeida, W.B.D., Guimarães, L., Nascimento Jr., C.S.: Theoretical investigation on the molecular inclusion process of urease inhibitors into p-sulfonic acid calix[4,6]arenes. Chem. Phys. Lett. 692, 117–123 (2018)
Crini, G.: Review: a history of cyclodextrins. Chem. Rev. 114, 10940–10975 (2014)
Shaikh, M., Swamy, Y.M., Pal, H.: Supramolecular host–guest interaction of acridine dye with cyclodextrin macrocycles: photophysical, pKa shift and quenching study. J. Photochem. Photobiol. A 258, 41–50 (2013)
Chen, M., Diao, G., Zhang, E.: Study of inclusion complex of β-cyclodextrin and nitrobenzene. Chemosphere 63, 522–529 (2006)
Kaanumalle, L.S., Gibb, C.L.D., Gibb, B.C., Ramamurthy, V.: A hydrophobic nanocapsule controls the photophysics of aromatic molecules by suppressing their favored solution pathways. J. Am. Chem. Soc. 127, 3674–3675 (2005)
Mukhopadhyay, M., Banerjee, D., Mukherjee, S.: Proton-transfer reaction of 4-methyl-2,6-diformyl phenol in cyclodextrin nanocage. J. Phys. Chem. A 110, 12743–12751 (2006)
Zhang, J., Ma, P.X.: Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv. Drug Deliv. Rev. 65, 1215–1233 (2013)
Trapani, A., Laurentis, N.D., Armenise, D., Carrieri, A., Defrenza, I., Rosato, A., Mandracchia, D., Tripodo, G., Salomone, A., Capriati, V., Franchini, C., Corbo, F.: Enhanced solubility and antibacterial activity of lipophilic fluoro-substituted N-benzoyl-2-aminobenzothiazoles by complexation with β-cyclodextrins. Int. J. Pharm. 497, 18–22 (2016)
Kfoury, M., Auezova, L., Greige-Gerges, H., Fourmentin, S.: Promising applications of cyclodextrins in food: improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 131, 264–272 (2015)
Assaf, K.I., Suckova, O., Danaf, N.A., Glasenapp, V.V., Gabel, D., Nau, W.M.: Dodecaborate functionalized anchor dyes for cyclodextrin-based indicator displacement applications. Org. Lett. 18, 932–935 (2016)
Aytac, Z., Kusku, S.I., Durgun, E., Uyar, T.: Quercetin/β-cyclodextrin inclusion complex embedded nanofibres: slow release and high solubility. Food Chem 197, 864–871 (2016)
Sayed, M., Pal, H.: Supramolecularly assisted modulations in chromophoric properties and their possible applications: an overview. J. Mater. Chem. C 4, 2685–2706 (2016)
ten Brummelhuis, N., Heilmann, M.T.: Polymerization of ternary inclusion complexes of interacting monomer pairs with γ-cyclodextrin. Macromolecules 18, 6879–6887 (2016)
Shinozaki, M., Sakai, M., Yamaguchi, S., Fujioka, T., Fujii, M.: S1–S0 Electronic spectrum of jet-cooled m-aminophenol. Phys. Chem. Chem. Phys. 5, 5044–5050 (2003)
Liebert, M.A.: Final report on the safety assessment of p-aminophenol, m-aminophenol, and o-aminophenol. J. Am. Coll. Toxicol. 7, 279–333 (1988)
Wang, J.L., Miao, F.M., Zhou, W.H., Ma, S.K.: Synthesis and structure of inclusion complex of cyclomaltoheptaose (β-cyclodextrin) with m-aminophenol. Chin. J. Chem. 20, 358–361 (2002)
Robinson, T.W., Kjaergaard, H.G., Ishiuchi, S., Shinozaki, M., Fujii, M.: Vibrational overtone spectroscopy of jet-cooled aminophenols as a probe for rotational isomers. J. Phys. Chem. A 108, 4420–4427 (2004)
Reese, J.A., Nguyen, T.V., Korter, T.M., Pratt, D.W.: Charge redistribution on electronic excitation. Dipole moments of cis- and trans-3-aminophenol in their S0 and S1 electronic states. J. Am. Chem. Soc. 126, 11387–11392 (2004)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven T., Montgomery, J.A., Jr. Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J.: Gaussian 09. Revision C.01, Gaussian, Inc., Wallingford CT, 2009
Gledening, E.D., Reed, A.E., Carpenter, J.A., Weinhold, F.: NBO Version 3.1
Warner, I.M., Mcgown, L.B.: Advances in Multidimensional Luminescence, vol. 2. JAI Press, Greenwisch (1993)
Agbaria, R.A., Butterfield, M.T., Warner, I.M.: Use of cyclodextrins and fluorescence spectroscopy to probe the dual fluorescence of 9-anthroic acid. J. Phys. Chem. 100, 17133–17137 (1996)
Oana, M., Tintaru, A., Gavrilliu, D., Maior, O., Hillebrand, M.: Spectral study and molecular modeling of the inclusion complexes of β-cyclodextrin with some phenoxathiin derivatives. J. Phys. Chem. B 106, 257–263 (2002)
El-Kemary, M.A., El-Gezawy, H.S.: Spectral study and global analysis of fluorescence decays of the inclusion complexes of 2-amino-4,6-dimethyl pyrimidine with α- and β-cyclodextrins. J. Photochem. Photobiol. A 155, 151–156 (2003)
Chen, Y., Xu, T., Shen, X., Gao, H.: Temperature dependence of the inclusion–dissociation behavior of the inclusion complexes between cationic substituted 3H-indoles and β-cyclodextrin. Design of a novel type of semi-rotaxane. J. Photochem. Photobiol. A 173, 42–50 (2005)
Meyer, M., Mialocq, J.C., Rougée, M.: Fluorescence lifetime measurements of the two isomers of the laser dye DCM. Chem. Phys. Lett. 150, 484–490 (1988)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Letts. 393, 51–57 (2004)
Zhao, Y., Truhlar, D.G.: The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor. Chem. Acc. 120, 215–241 (2008)
Boys, S.F., Barnardi, F.: The calculation of small molecular interactions by the difference of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970)
Domingo, L.R., Ríos-Gutiérrez, M., Pérez, P.: Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21, 1–22 (2016)
Chhattaraj, P.K., Sarkar, U., Roy, D.R.: Electrophilicity index. Chem. Rev. 106, 2065–2091 (2006)
Nora, M., Fatiha, M., Leila, N., Sakina, H., Eddine, K.D.: Density functional study of inclusion complex of Albendazole/cucurbit [7]: structure, electronic properties, NBO, GIAO and TD-DFT analysis. J. Mol. Liq. 211, 40–47 (2015)
Carlson, B.C., Keller, J.M.: Orthogonalization procedures and the localization of Wannier functions. Phys. Rev. 105, 102–103 (1957)
Carpenter, J.E., Weinhold, F.: Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure. J. Mol. Struct. (Theochem.) 169, 41–62 (1988)
Reed, A.E., Weinstock, R.B., Weinhold, F.: Natural population analysis. J. Chem. Phys. 83, 735–739 (1985)
Davidson, E.R.: Reduced Density Matrices in Quantum Chemistry, 1st edn. Academic Press, New York (1976)
Acknowledgements
SS thanks Prof. Samita Basu (Chemical Sciences Division, Saha Institute of Nuclear Physics, Kolkata—700 064, India) for her valuable scientific suggestions and Mr. Ajay Das (Chemical Sciences Division, Saha Institute of Nuclear Physics, Kolkata—700 064, India) for fluorescence lifetime measurements. Also, SS sincerely acknowledges Prof. Pranab Sarkar, Dr. Md. Motin Seikh (Department of Chemistry, Siksha Bhavana, Visva-Bharati, Santiniketan—731 235, India) and Dr. Nilanjan Bondyopadhaya (ISERC, Siksha Bhavana, Visva-Bharati, Santiniketan—731 235, India) for helping in DFT calculations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Majhi, K., Bandyopadhyay, P., Khatun, R. et al. Prediction of the most preferable rotamer of meta-aminophenol in β-cyclodextrin cavity in aqueous medium by using spectroscopic and DFT computational studies. J Incl Phenom Macrocycl Chem 97, 77–86 (2020). https://doi.org/10.1007/s10847-020-00985-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-00985-0