Abstract
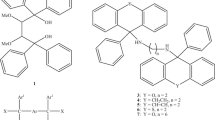
The inclusion behaviour of compounds N,N′-bis(9-cyclohexyl-9-xanthenyl)ethylenediamine (OED) and N,N′-bis(9-cyclohexyl-9-thioxanthenyl)ethylenediamine (SED) was assessed in the presence of pyridine (PYR) and its three methylpyridine isomers (2MP, 3MP and 4MP). PYR, 3MP and 4MP were each enclathrated by OED when it was recrystallized independently from each guest solvent, but failed to include 2MP. The thio host derivative, SED, was less efficient, forming a complex only with PYR. When these guests were mixed in equimolar amounts and each host recrystallized from the mixture, OED constantly displayed a significantly enhanced preference for 4MP (near-complete in many instances), while complexation failed under these circumstances for SED, even when PYR was present in the guest mixture (despite PYR having been included in the single solvent experiment). A selectivity order of 4MP (92.8%) ≫ PYR (6.0%) > 3MP (0.9%) > 2MP (0.3%) was noted for OED when it was recrystallized from the equimolar quaternary mixed solvent system. The selectivity of OED towards 4MP was investigated using single crystal diffraction (SCXRD) and thermal (TA) analyses: interestingly, only 4MP experienced a strong classical hydrogen bond with OED, in direct relation to the enhanced selectivity of OED for 4MP while, additionally, this complex displayed an increased thermal stability relative to the other two complexes with OED.







Similar content being viewed by others
References
Cram, D.J.: The design of molecular hosts, guest, and their complexes. Angew. Chem. Int. Ed. Engl. 27, 1009–1020 (1988)
Atwood, J.L., Gokel, G.W., Barbour, L.J.: Comprehensive Supramolecular Chemistry II. Elsevier, Oxford (2017)
Steed, J.W., Atwood, J.L.: Supramolecular Chemistry. Wiley, Chichester (2009)
Kamp, R.M., Kyriakidis, D., Choli-Papadopoulou, T.: Proteome and Protein Analysis. Springer, Berlin (2000)
Silwa, W., Kozlowski, C.: Calixarenes and Resorcinarenes: Synthesis, Properties and Applications. Wiley, Weinheim (2009)
Asfari, Z., Böhmer, V., Harrowfield, Z., Vicens, J.: Calixarenes 2001. Kluwer Academic Publishers, Dordrecht (2001)
Heaton, C.A.: An Introduction to Industrial Chemistry. Blackie Academic & Professional, New York (1991)
Scriven, E.F.V.: Pyridines: From Lab to Production. Academic Press, London (2013)
Larrañaga, M.D., Lewis, R.J., Lewis, R.A.: Hawley’s Condensed Chemical Dictionary. Wiley, New York (2016)
Bacsa, J., Caira, M.R., Jacobs, A., Nassimbeni, J.R., Toda, F.: Complexation with diol host compounds. Part 33. Inclusion and separation of pyridines by a diol host compound. Cryst. Eng. 3, 251–261 (2000)
Weber, E., Nitsche, S., Wierig, A., Csöregh, I.: Inclusion compounds of diol hosts featuring two 9-hydroxy-9-fluorenyl or analogous groups attached to linear spacer units. Eur. J. Org. Chem. 2002, 856–872 (2002)
Nassimbeni, L.R., Ramon, G., Weber, E.: Inclusion by a fluorenyl diol host with substituted pyridines: structures, selectivity and kinetics of desorption. J. Therm. Anal. Calorim. 90, 31–37 (2007)
Barton, B., Caira, M.R., Hosten, E.C., McCleland, C.W.: A computational, X-ray crystallographic and thermal stability analysis of TETROL and its pyridine and methylpyridine inclusion complexes. Tetrahedron 69, 8713–8723 (2013)
Barton, B., Hosten, E.C., Jooste, D.V.: Comparative investigation of the inclusion preferences of optically pure versus racemic TADDOL hosts for pyridine and isomeric methylpyridine guests. Tetrahedron 73, 2662–2673 (2017)
Barton, B., de Jager, L., Hosten, E.C.: Host proficiency of N,N′-bis(9-phenyl-9-thioxanthenyl)ethylenediamine for pyridine and the methylpyridine guests—a competition study. Supramol. Chem. 30, 61–71 (2018)
Barton, B., Senekal, U., Hosten, E.C.: Compounds N, N’-bis(9-cyclohexyl-9-xanthenyl)ethylenediamine and its thio derivative, N, N’-bis(9-cyclohexyl-9-thioxanthenyl)ethylenediamine, as potential hosts in the presence of xylenes and ethylbenzene: Conformational analyses and molecular modelling considerations. Tetrahedron 75, 3399–3412 (2019)
Barton, B., Caira, M.R., McCleland, C.W., Taljaard, B.: Synthesis of N,N’-bis(9-phenylxanthen-9-yl)ethylenediamine and an investigation of its host‒guest inclusion potential. J. Chem. Soc. Perkin Trans. 2(4), 865–869 (2000)
Bruker: APEX2, SADABS and SAINT. Bruker AXS Inc., Madison (2010)
Sheldrick, G.M.: SHELXT – Integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015)
Sheldrick, G.M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015)
Hübschle, C.B., Sheldrick, G.M., Dittrich, B.: ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44, 1281–1284 (2011)
Macrae, C.F., Bruno, I.J., Chisholm, J.A., Edgington, P.R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J., Wood, P.A.: Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 41, 466–470 (2008)
Nassimbeni, L.R.: Physicochemical aspects of host-guest compounds. Acc. Chem. Res. 36, 631–637 (2003)
Acknowledgements
Financial support is acknowledged from the Nelson Mandela University and the National Research Foundation (NRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Senekal, U., Barton, B. & Hosten, E.C. Inclusion ability and selectivity of ethylenediamine derivatives for pyridine in the presence of methylpyridine isomers. J Incl Phenom Macrocycl Chem 96, 251–262 (2020). https://doi.org/10.1007/s10847-019-00966-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-019-00966-y