Abstract
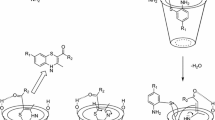
β-Cyclodextrin (β-CD) is a versatile and naturally occurring host which strongly binds adamantane and its derivatives. Based on this binding, we prepared a host–guest inclusion complex of β-CD with adamantane, and selectively iodinated the included adamantane to 1-iodoadamantane by iodoform. The only formed monoiodo product confirmed our initial assumption that three out of the four methine groups of the guest adamantane are shielded when adamantane is included into the cavity of β-CD and, therefore, iodination just takes places on the methine group directing to the outside of the cavity of β-CD.
Graphical Abstract




Similar content being viewed by others
References
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry. Springer, Berlin (1978)
Easton, C.J., Onagi, H.: Cyclodextrins as molecular reactors. In: Brinker, U.H., Mieusset, J.-L. (eds.) Molecular Encapsulation- Organic Reactions in Constrained Systems, pp. 71–89. Wiley, Chichester (2010)
Van Etten, R.L., Sebastian, J.F., Clowes, G.A., Bender, M.L.: Acceleration of phenyl ester cleavage by cycloamyloses. A model for enzymic specificity. J. Am. Chem. Soc. 89, 3242–3253 (1967)
Breslow, R., Dong, S.D.: Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 98, 1997–2012 (1998)
Komiyama, M., Monflier, E.: Cyclodextrin catalysis. In: Dodziuk, H. (ed.) Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications, pp. 93–105. Wiley, Weinheim (2006)
Mansoori, G.A., de Araujo, P.L.B., de Araujo, E.S.: Diamondoid Molecules. World Scientific Publishing Co., Hackensack (2012)
Mansoori, G.A.: Diamondoids molecules. In: Rice, S.A. (ed.) Advances in Chemical Physics, pp. 207–258. Wiley, Hoboken (2007)
Ashfold, M.N.R., May, P.W., Rego, C.A., Everitt, N.M.: Thin film diamond by chemical vapour deposition methods. Chem. Soc. Rev. 23, 21–30 (1994)
Ramazani, H., Mansoori, G.A.: Diamondoids as molecular building blocks for nanotechnology. In: Mansoori, G.A., George, T.F., Assoufid, L., Zhang, G. (eds.) Molecular Building Blocks for Nanotechnology: From Diamondoids to Nanoscale Materials and Applications, pp. 44–71. Springer, New York (2007)
Schwertfeger, H., Fokin, A.A., Schreiner, P.R.: Diamonds are a chemist’s best friend: diamondoid chemistry beyond adamantine. Angew. Chem. Int. Ed. 47, 1022–1036 (2008)
Voskuhl, J., Waller, M., Bandaru, S., Tkachenko, B.A., Fregonese, C., Wibbeling, B., Schreiner, P.R., Ravoo, B.J.: Nanodiamonds in sugar rings: an experimental and theoretical investigation of cyclodextrin–nanodiamond inclusion complexes. Org. Biomol. Chem. 10, 4524–4530 (2012)
Gelb, R.I., Schwartz, L.M.: Complexation of adamantane-ammonium substrates beta-cyclodextrin and its O-methylated derivatives. J. Inclusion Phenom. Mol. Recognit. Chem. 7, 537–543 (1989)
Weickenmeier, M., Wenz, G.: Cyclodextrin sidechain polyesters-synthesis and inclusion of adamantan derivatives. Macromol. Rapid Commun. 17, 731–736 (1996)
Stauss, S., Terashima, K.: Diamondoids: Synthesis, Properties, and applications. Pan Standford Publishing, Singapore (2017)
Wanka, L., Iqbal, K., Schreiner, P.R.: The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem. Rev. 113, 3516–3604 (2013)
Schreiner, P.R., Lauenstein, O., Butova, E.D., Fokin, A.A.: The first efficient iodination of unactivated aliphatic hydrocarbons. Angew. Chem. Int. Ed. 38, 2786–2788 (1999)
Del Valle, E.M.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Jaime, C., Redondo, J., Sanchez-Ferrando, F., Virgili, A.: β-Cyclodextrin inclusion complex with adamantane Intermolecular 1H 1H NOE determinations and molecular mechanics calculations. J. Mol. Struct. 15, 317–329 (1991)
Acknowledgements
We would like to thank Hakim Sabzevari University for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezaei-Seresht, E., Rahmandoost, M. & Mahdavi, B. Green and selective iodination of diamondoid adamantane by β-cyclodextrin as a molecular reactor. J Incl Phenom Macrocycl Chem 95, 51–54 (2019). https://doi.org/10.1007/s10847-019-00914-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-019-00914-w