Abstract
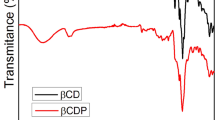
Rotaxanes and pseudorotaxanes are the supramolecular objects that attract much attention due to their low toxicity, sliding, dethreading and easy modification. Thus, polyrotaxanes and polypseudorotaxanes can be considered as components in drug delivery systems, sensor devices, implants, contrasting agents, fluorescent probes in other diagnostic systems. Therefore, we have prepared the pseudorotaxanes based on the β-cyclodextrin (β-CD) and molecule-“guest”—polyoxypropylenedimethacrylate (POPDMA) as carrier of end-capped methacrylate groups. The presence of such groups allows pseudorotaxane to be co-polymerized with acrylamide and methylene-bis-acrylamide and to develop cross-linked polymer matrices, which implies their further investigation as systems for a drug release. The structure of these substances was confirmed by FTIR- and NMR-spectroscopy, differential scanning calorimetry (DSC), X-ray analysis [wide-angle X-ray scattering (WAXS)], pyrolysis mass spectrometry. The ratio of POPDMA to β-CD was found to be 1:3, according to NMR data. The interactions between β-CD and POPDMA in the aqueous solution and in the dry mechanical mixture are entirely different, due to formation of pseudorotaxane. The results obtained by pyrolysis mass-spectrometry, WAXS and DSC well correlate with mechanism of formation of inclusion complexes, involving a linear molecule and cyclodextrins as described earlier.









Similar content being viewed by others
Abbreviations
- β-CD:
-
β-Cyclodextrin
- MW:
-
Molecular weight
- MetA:
-
Methacrylic anhydride
- PPG:
-
Polyoxypropylene glycol
- ТЕА:
-
Triethylamine
- POPDMA:
-
Polyoxypropylenedimethacrylate
- WAXS:
-
Wide angle X-ray scattering
- DSC:
-
Differential scanning calorimetry
- TGA:
-
Thermogravimetric analysis
References
Mattia, E., Otto, S.: Supramolecular systems chemistry. Nat. Nanotechnol. 10, 111–119 (2015)
Harada, A., Takashima, Y., Yamaguchi, H.: Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 38, 875–882 (2009)
Harada, A., Okada, M., Li, J., Kamachi, M.: Preparation and characterization of inclusion complexes of poly(propy1ene glycol) with cyclodextrins. Macromolecules 28, 8406–8411 (1995)
Lehn, J.-M.: Supramolecular Chemistry: Concepts and Perspectives, p. 353. Wiley, Hoboken (2011)
Girek, T.: Cyclodextrin-based rotaxanes. J. Incl. Phenom. Macrocycl. Chem. 74, 1–21 (2012)
Harada, А, Hashidzume, А, Takashima, Y.: Cyclodextrin-based supramolecular polymers. Adv. Polym. Sci. 201, 1–43 (2006)
Zhanga, J., Mab, P.X.: Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv. Drug. Deliv. Rev. 65, 1–39 (2013)
Harada, A., Li, j, Kamachi, M.: Double-stranded inclusion complexes of cyclodextrin threaded on poly(ethylene glycol). Nature 370, 126–128 (1994)
Yui, N., Katoono, R., Yamashita, A.: Functional cyclodextrin polyrotaxanes for drug delivery. Adv. Polym. Sci. 222, 55–77 (2009)
Panova, I.G.. Topchieva, I.N.: Rotaxanes and polyrotaxanes. Their synthesis and the supramolecular devices based on them. Russ. Chem. Rev. 70, 28–51 (2001) (in Russian)
Sliwa, W., Girek, T.: Cyclodextrins: Properties and Applications. Wiley-VCH, Weinheim (2017)
Manakker, F., Vermonden, T., Nostrum, C.F., Hennink, W.E.: Cyclodextrin-based polymeric materials: synthesis, properties and pharmaceutical/biomedical applications. Am. Chem. Soc. 10, 3157–3175 (2009)
Harada, A., Hashidzume, A., Yamaguchi, H., Takashima, Y.: Polymeric rotaxanes. Chem. Rev. 109, 5974–6023 (2009)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery. AAPS PharmSciTech 6, 329–357 (2005)
Otero-Espinar, F.J., Torres-Labandeira, J.J., Alvarez-Lorenzo, C., Blanco-Mundez, J.: Cyclodextrins in drug delivery systems. J. Drug Deliv. Sci. Technol. 20, 289–301 (2010)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1025 (1996)
Li, J.J., Zhao, F., Li, J.: Polyrotaxanes for applications in life science and biotechnology. Appl. Microbiol. Biotechnol. 90, 427–443 (2011)
Wenz, G., Han, B.H., Muller, A.: Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 106, 782–817 (2006)
Zheng, Y., Wyman, I.: Supramolecular nanostructures based on cyclodextrin and poly(ethylene oxide): syntheses, structural characterizations and applications for drug delivery. Polymers 8, 190–198 (2016)
Ohga, K., Takashima, Y., Takahashi, H., Kawaguchi, Y., Yamaguchi, H., Harada, A.: Preparation of supramolecular polymers from a cyclodextrin dimer and ditopic guest molecules: control of structure by linker flexibility. Macromolecules 38, 5897–5904 (2005)
Harada, A., Li, J., Nakamitsu, T., Kamachi, M.: Preparation and characterization of polyrotaxanes containing many threaded α-cyclodextrins. J. Org. Chem. 58, 7524–7528 (1993)
Joung, Y.K., Park, H.D., Yui, N., Park, K.D.: Supramolecular structures with cyclodextrins for biomedical applications. Biomater. Res. 11, 162–169 (2007)
Pozuelo, J., Mendicuti, F., Mattice, W.L.: Inclusion complexes of chain molecules with cycloamyloses III. molecular dynamics simulations of polyrotaxanes formed by poly(propylene glycol) and β-cyclodextrins. Polym. J. 30, 479–484 (1998)
Riabov, S.V., Boyko, V.V., Bortnytskyy, V.I., Dmytriyeva, T.V., Kobrina, L.V., Kercha, Y.Y.: Mass-spectrometric studies of inclusion complexes of silylation derivative of β-cyclodextrin with organic compounds obtained in the aqueous environment. Ukr. Chem. J. 75, 58–63 (2009) (in Ukrainian)
Goodacre, R., Kell, D.B.: Pyrolysis mass spectrometry and its applications in biotechnology. Curr. Opin. Biotechnol. 7, 20–28 (1996)
Author information
Authors and Affiliations
Contributions
LO was engaged in the synthesis of samples and their identification by NMR spectroscopy. LK performed studying of samples by DSC and TGA. SS elaborated and provided synthetic procedures for monomers and cross-linked polymers. VB carried out pyrolysis mass spectrometry research VD studied the samples by WAXS method and analyzed the data obtained. SR took part in the FT-IR, DCS and TGA investigations, summarized all data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Orel, L., Kobrina, L., Sinelnikov, S. et al. β-Cyclodextrin-containing pseudorotaxanes as building blocks for cross-linked polymers. J Incl Phenom Macrocycl Chem 92, 273–280 (2018). https://doi.org/10.1007/s10847-018-0838-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0838-5