Abstract
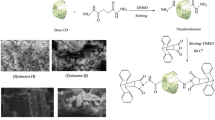
In the present work, new bis hexachloro methanoisoindol derivatives (1) and (3) were prepared. Also, novel interlocked compounds [2] rotaxane (2) and [3] rotaxane (4) have been synthesized. [2] rotaxane (2) was formed by threading of β-cyclodextrin into succinic dihydrazide to give pseudorotaxane that was trapped by using chlorendic anhydride. [3] rotaxane (4) was obtained by entering α-cyclodextrin rings through sebacoyl chloride to afford the corresponding pseudorotaxane and locked in the terminal groups by using N-amino-1,4,5,6,7,7-hexachloro-5-norbornene-2,3-dicarboximide. The chemical structure of the obtained compounds was confirmed by FT-IR, 1H-NMR, 13C-NMR and 2D-NMR (cosy) spectra. The morphology of [2] and [3] rotaxanes was examined by Scanning Electron Microscope.













Similar content being viewed by others
References
Bruns, C.J., Stoddart, J.F.: The Nature of the Mechanical Bond From Molecules to Machines. Wiley, Hoboken (2017)
Izatt, R.M.: Macrocyclic and Supramolecular Chemistry, How Izatt-Christensen Award Winners Shaped the Field, Wiley, Hoboken (2016)
Lewis, J.E.M., Beer, P.D., Loeb, S.J., Goldup, S.M.: Metal ions in the synthesis of interlocked molecules and materials. Chem. Soc. Rev. 46, 2577–3259 (2017)
Dardeer, H.M., Hassan, M.A.: Synthesis of [2] rotaxanes derived from host-gust interaction. Int. J. Chem. 7(1), 161–167 (2015)
Dardeer, H.M.: Formation of new inclusion complexes depend on cyclodextrin. Chem. J. 5(1), 14–19 (2015)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Loftsson, T., Sigursson, H.H., Másson, M., Schipper, N.: Preparation of solid drug/cyclodextrin complexes of acidic and basic drugs. Pharmazie 95, 25–29 (2004)
Dardeer, H.M.: Importance of cyclodextrins into inclusion complexes. Int. J. Adv. Res. 2(4), 414–428 (2014)
Hiratani, K., Suga, J.-I., Nagawa, Y., Houjou, H., Tokuhisa, H., Numata, M., Watanabe, K.: A new synthetic method for rotaxanes via tandem Claisen rearrangement, diesterification, and aminolysis. Tetrahedron Lett. 43, 5747 (2002)
Hiratani, K., Kaneyama, M., Nagawa, Y., Koyama, Y., Kanesato, M.: Synthesis of [1] rotaxane via covalent bond formation and its unique fluorescent response by energy transfer in the presence of lithium ion. J. Am. Chem. Soc. 126, 13568 (2004)
Kameta, N., Hiratani, K., Nagawa, Y.: A novel synthesis of chiral rotaxanes via covalent bond formation. Chem. Commun. 4, 466 (2004)
Hirose, K., Nishihara, K., Harada, N., Nakamura, Y., Masuda, D., Araki, M., Tobe, Y.: Highly selective and high-yielding rotaxane synthesis via aminolysis of prerotaxanes consisting of a ring component and a stopper unit. Org. Lett. 9, 2969 (2007)
Steemers, L., Wanner, M.J., Ehlers, A.W., Hiemstra, H., van Maarseveen, J.H.: A short covalent synthesis of an all-carbon-ring [2]rotaxane. Org. Lett. 19, 2342–2345 (2017)
Harrison, I.T., Harrison, S.: Synthesis of a stable complex of a macrocycle and a threaded chain. J. Am. Chem. Soc. 89, 5723–5724 (1967)
Chill, G., Zollenkoof, H.: Chapter I Pseudorotaxanes and Rotaxanes. Nachr. Chem. Tech. 79, 149–152 (1967)
Johnston, A.G., Leigh, D.A., Pritchard, R.J., Deegan, M.D.: Angew. Chem. Int.Ed. 34, 1209–1212 (1995)
Yamakawa, T., Nishimura, S.: Liquid formulation of a novel nonfluorinated topical quinolone, T-3912, utilizing the synergic solubilizing effect of the combined use of magnesium ions and hydroxypropyl-β-cyclodextrin. J. Control. Rel. 86, 101–113 (2003)
Weck, M., Mohr, B., Sauvage, J.P., Grubbs, R.H.: Synthesis of catenane structures via ring-closing metathesis. J. Org.Chem. 64, 5463–5471 (1999)
Anelli, P.-L., Spencer, N., Stoddart, J.F.: A Molecular Shuttle. J. Am. Chem. Soc. 113, 513–5133 (1991)
Maksimov, M.O., Pan, S.J., Link, A.J.: Lasso peptides: structure, function, biosynthesis, andengineering. Nat. Prod. Rep. 29, 996–1006 (2012)
Potterat, O., Wagner, K., Gemmecker, G., Mack, J., Puder, C., Vettermann, R., Streicher, R.: BI-32169, a bicyclic 19-peptide with strong glucagon receptor antagonist activity from Streptomyces sp.. J. Nat. Prod. 67, 1528–1531 (2004)
Yang, W., Li, Y., Liu, H., Chi, L., Li, Y.: Design and assembly of rotaxane-based molecular switches and machines. Small 8, 504 (2012)
Balzani, V., Semeraro, M., Venturi, M., Credi, A.: Reading and powering molecular machines by light. In: Feringa, B. L., Browne, W. R. (eds.) Molecular Switches, 2nd Ed., vol. 2, p. 597. Wiley-VCH, Weinheim (2011)
Bodis, P., Yeremenko, S., Berna, J., Buma, W.J., Leigh, D.A., Woutersen, S.: Bimodal dynamics of mechanically constrained hydrogen bonds revealed by vibrational photon echoes. J. Chem. Phys. 134, 134504 (2011)
Lussis, P., Svaldo-Lanero, T., Bertocco, A., Fustin, C.-A., Leigh, D.A., Duwez, A.-S.: A single synthetic small molecule that generates force against a load. Nat. Nanotechnol. 6, 553 (2011)
Yan, X., Zhou, M., Chen, J., Chi, X., Dong, S., Zhang, M., Ding, X., Yu, Y., Shao, S., Huang, F.: Supramolecular polymer nanofibers via electrospinning of a heteroditopic monomer. Chem. Commun. 47, 7086 (2011)
Niu, Z., Huang, F., Gibson, H.W.: Supramolecular AA−BB-type linear polymers with relatively high molecular weights via the self-assembly of bis (m-phenylene)-32-crown-10 cryptands and a bisparaquat derivative. J. Am. Chem. Soc. 133, 133 (2011)
Tuncel, D., Uenal, O., Artar, M.: Supramolecular assemblies constructed by cucurbituril-catalyzed click reaction. Isr. J. Chem. 51, 525 (2011)
Vedernikov, A.I., Lobova, N.A., Kuzmina, L.G., Howard, J.A.K., Strelenko, Y.A., Alfimov, M.V., Gromov, S.P.: Pseudorotaxane complexes between viologen vinylogues and cucurbit [7] uril: new prototype of photocontrolled molecular machine. J. Mol. Struct 989, 114 (2011)
Briggs, B.N., Durola, F., McMillin, D.R., Sauvage, J.-P.: Luminescence studies of copper (I)-containing [2] pseudorotaxanes. Can. J. Chem. 89, 98 (2011)
Niu, Z., Slebodnick, C., Gibson, H.W.: Pseudocryptand-type [3] Pseudorotaxane and “hook-ring” polypseudo [2] catenane based on a bis (m-phenylene)-32-crown-10 derivative and bisparaquat derivatives. Org. Lett. 13, 4616 (2011)
Fernando, I.R., Bairu, S.G., Ramakrishna, G., Mezei, G.: Single-color pseudorotaxane-based temperature sensing. New J. Chem. 34, 2097 (2010)
Li, J.J., Zhao, F., Li, J.: Polyrotaxanes for applications in life science and biotechnology. Appl. Microbiol. Biotechnol. 90, 427 (2011)
Balzani, V., Credi, A., Venturi, M.: Molecular Devices and Machines—A Journey Into the Nano World. Wiley-VCH, Weinheim (2003)
Dardee, H.M., El-sisi, A.A., Emam, A.A., Nora, M., Hilal: Synthesis, application of a novel azo dye and its inclusion complex with beta-cyclodextrin onto polyester fabric. Int. J. Text. Sci. 6(3), 79–87 (2017)
Acknowledgements
The author is grateful to her father, Prof. Aly of organic chemistry and her family.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dardeer, H.M. Synthesis, characterization of novel rotaxanes depend on cyclodextrins. J Incl Phenom Macrocycl Chem 91, 105–114 (2018). https://doi.org/10.1007/s10847-018-0805-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0805-1