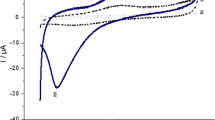
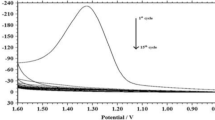
Abstract
Cyclic voltammetry was used to detect p-sulfonated calix[n]arenas (SCnA) how to immobilize on gold surface. P-sulfonated calix[n]arenes not only increased the specific surface area of the modified electrode, but also improved the enrichment ability of tyrosine. This led to a significant increase in peak current, and improved the sensitivity of tyrosine determination on the p-sulfonated calix[n]arenes-modified electrode. The modified electrode showed good catalytic ability of l-tyrosine oxidation reaction. The peak current of l-tyrosine increased and the oxidation peak potential shifted negatively with cavity size of the SCnA-modified electrode, which indicated that the catalytic ability of the modified electrode to l-tyrosine oxidation reaction was also enhanced. For the tyrosine guest molecule, the order of electrochemical activity and magnitude of catalytic ability of the oxidation reaction of the three modified gold electrodes was as follows: SC4A > SC6A > SC8A. The electrode had high selectivity and stability for the determination of tyrosine, with a wide linear range, low detection limit and high sensitivity at different concentrations under different pH values. This resulted in an accurate, fast, sensitive electrochemical method for the determination of tyrosine. The sensor was used for the determination of tyrosine in human urine with satisfactory results.








Similar content being viewed by others
References
Leon, L.J., Pratt, C.C., Vasquez, L.J., Weers, P.M.: Tyrosine fluorescence analysis of apolipophorin III-lipopolysaccharide interaction. Arch Biochem Biophys. 1, 38–45 (2006)
Gao, X.Y., Zhang, K.G., Kong, X.X., You, X.X.: Determination of tryptophan and tyrosine in four edible wild plants by molecular fluorescence spectrometry. Food Sci. 33, 231–234 (2012)
Gleason, N.J., Vostrikov, V.V., Greathouse, D.V., Grant, C.V., Opella, S.J., Koeppe, R.E.: Tyrosine replacing tryptophan as an anchor in GWALP peptides. Biochemistry 51, 2044–2053 (2012)
Gao, C.Y., Fan, S.H.: Determination of tyrosine and tryptophan by sequential injection analysis and chemiluminescence detection. Anal. Lett. 47, 178–189 (2014)
Sayed, Y.K.: Quenching effect of l-tyrosine on peroxyoxalate chemiluminescence of berberine as the fluorophore. J. Iran. Chem. Soc. 10, 915–920 (2013)
Letellier, S., Garnier, J.P., Spy, J.: Determination oft he DOPA/l-tyrosine ratio in human plasma by high performance liquid chromatography: usefulness as a marker in metastatic malignant melanoma. J. Chromato. B. 696, 9–17(1997)
Li, X., Chen, Z., Yang. F., Pan, J., Li, Y.: Development of a microchip-pulsed electrochemical method for rapid determination of L-DOPA and tyrosine in Mucuna pruriens. J. Sep. Sci. 36, 1590–1596 (2013)
Chen, H., Wang, X., Chopra, S., Adams, E., Schepdael, A.V.: Development and validation of an indirect pulsed electrochemical detection method for monitoring the inhibition of Abl1 tyrosine kinase. J. Pharm. Biomed. Anal. 90, 52–57 (2014)
Stödeman, M., Dhar, N.: Microcalorimetric titration of a tetra-sulfonated calix[4]arene with alkylammonium ions in aqueous solution. J. Chem. Soc. Faraday. Trans. 94, 899–903 (1998)
Bonal, C., Israe, L.Y., Morel, J.P.: Binding of inorganic and organic cations by p-sulfonato calix[4]arene in water, a thermodynamic study. Chem. Soc. Perkin. Trans. 2, 1075–1078 (2001)
Shinkai, S., Araki, K., Manabe, O.: NMR determination of association constants for calixarene complexes. Evidence for the formation of a 1, 2 complex with calix[8]arene. J. Am. Chem. Soc. 110, 7214–7215 (1988)
Liu, Y., Wang, L.H., Guo, D.S.: Thermodynamics of interactions between organic ammonium ions and sulfonatocalixarenes. Thermochim. Acta. 443,132–135(2006)
Zhang, J., Guo, D.S., Liu, Y.: Solid-state supramolecular architecturesby p-sulfonatocalix[4] arene with bispyridinium derivatives. Chin. J. Chem. 28, 1575–1579 (2010)
Guo, D.S., Wang, K., Wang, Y.X., Liu, Y.: Cholinesterase-responsive supramolecular vesicle. J. Am. Chem. Soc. 134, 10244–10250 (2012)
Pang, T.T., Cai, Z.F., Du, L.M., Guo, M.D., Fu, Y.L.: Determination of tryptophan using a p-sulfonated calix [4,6,8]arene modified gold electrode. Anal. Lett. 47, 1808–1820 (2014)
Pang, T.T., Du, L.M., Liu, H.L., Fu, Y.L.: Supeamolecular p-sulfonatedcalix[4,6,8]arene for tryptophan detection. Can. J. Chem. 92, 1139–1144 (2014)
Hassen, W.M., Abdelghani, A., Vonna, L., Cherif, K., Boussaid, M., Maaref, M.A.: Electrochemical proprieties and topology of gold electrodes with adsorbed penicillin G for biosensor applications. Sens. Actuators B. 120, 621–627 (2007)
Pan, D. W., Chen, J. H., Nie, L. H., Tao, W. Y., Yao, S. Z.: Amperometric glucose biosensor based on immobilization of glucose oxidase in electropolymerized o-aminophenol film at Prussian blue-modified platinum electrode. Electrochim. Acta. 49, 795–801 (2004)
Rajakannu, P., Elumalai, P., Hussain, F., Sathiyendiran, M.: Rhenium-based bicyclic supramolecule with calixarene-shaped bowls. J. Organomet. Chem. 725, 1–4 (2013)
Miller, J. N., Miller, J. C.: Statistics and Chemometrics for Analytical Chemistry, 4th ed., pp. 122. Pearson Education Limited, Chichester (2000)
Acknowledgements
This work was supported by the Doctoral Scientific Research Foundation of shanxi Province (No. 050502070397), Shanxi Province Natural Science Fund (No. 2012011008-3), Shanxi Province Natural Science Fund (No. 2014021018-3). Helpful suggestions by anonymous referees are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pang, TT., Zhang, XY. & Xue, YB. Determination of tyrosine using p-sulfonated calix[n]arene modified electrode. J Incl Phenom Macrocycl Chem 87, 275–282 (2017). https://doi.org/10.1007/s10847-017-0697-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-017-0697-5