Abstract
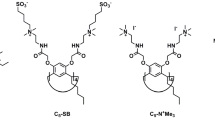
Cysteine-linked cyclophane dimer having two rhodamine moieties (2) was synthesized as a reduction-responsive host. Owing to self-quenching property of the two rhodamine moieties, cyclophane dimer 2 showed weak fluorescence intensity relative to that of the rhodamine B moiety itself. The cleavage of disulfide bond of 2 was performed by a treatment with reducing agents such as dithiothreitol, to give the corresponding monomeric cyclophanes having a rhodamine moiety. Such reductive degradation of 2 was detected by the increase on fluorescence intensity. As a host, cyclophane dimer 2 was found to show a stronger guest-binding affinity than the monomeric cyclophanes due to concentration effects of the macrocycles. In addition, reduction-responsive release of entrapped guest molecules by 2 was also monitored by fluorescence spectroscopy.









Similar content being viewed by others
Notes
6: MALDI-TOF MS (positive mode); m/z 1374 [M–Cl]+, where M shows C79H97ClN12O8S as a triamine derivative of cyclophane (free amine).
It was reported as difference fluorescence intensity between the presence and absence of the guests, because of emission originating from rhodamine-hosts with the excitation wavelength at 326 nm (TNS) and 318 nm (2,6-ANS).
References
Rebek, J. Jr.: Host–guest chemistry of calixarene capsules. Chem. Commun. 637–643 (2000)
Seward, E., Hopkins, R. B., Sauerer, W., Tam, S.-W., Diederich, F.: Redox-dependent binding ability of a flavin cyclophane in aqueous solution: hydrophobic stacking versus cavity-inclusion complexation. J. Am. Chem. Soc. 112, 1783–1790 (1990)
Nunez-Dallos, N., Reyes, A., Quevedo, R.: Hydrogen bond assisted synthesis of azacyclophanes from l-tyrosine derivatives. Tetrahedron Lett. 53, 530–534 (2012)
Hayashida, O., Nakamura, Y.: Synthesis of water-soluble cyclophane pentamers using click chemistry as a multivalent host for daunorubicin and doxorubicin. Bull. Chem. Soc. Jpn. 86, 223–229 (2013)
Slavik, J.: Anilinonaphthalene sulfonate as a probe of membrane composition and function. Biochem. Biophys. Acta. 694, 1–25 (1982)
Le Poul, N., Le Mest, Y., Jabin, I., Reinaud, O: Supramolecular modeling of mono-copper enzyme active sites with calix[6]arene-based funnel complexes. Acc. Chem. Res. 48, 2097–2106 (2015)
Carter, T.S., Mooibroek, T.J., Stewart, P.F. N., Crump, M.P., Galan, M.C., Davis, A.P.: Platform synthetic lectins for divalent carbohydrate recognition in water. Angew. Chem. Int. Ed. 55, 9311–9315 (2016)
Ogoshi, T., Yamagishi, T.: Pillararenes: versatile synthetic receptors for supramolecular chemistry. Eur. J. Org. Chem. 2961–2975 (2013)
Hayashida, O., Kojima, K.: Entrapment and release of guest molecules by reduction-responsive cyclophane dimers based on disulfide linkage. Bull. Chem. Soc. Jpn. 88, 729–735 (2015)
Nalluri, S.K.M., Voskuhi, J., Bultema, J. B., Boekema, E.J., Ravoo, B.J.: Light-responsive capture and release of DNA in a ternary supramolecular complex. Angew. Chem. Int. Ed. 50, 9747–9751 (2011)
Zhang, M., Zheng, B., Huang, F.: Synthesis of a four-armed cage molecule and its pH-controlled complexation with paraquat. Chem. Commun. 47, 10103–10105 (2011)
Yu, G., Han, C., Zhang, Z., Chen, J., Yan, X., Zheng, B., Liu, S., Huang, F.: Pillar[6]arene-based photoresponsive host–guest complexation. J. Am. Chem. Soc. 134, 8711–8717 (2012)
Wang, H., Wang, P., Xing, H., Li, N., Ji, X.: A multistimuli-responsive supramolecular polymer constructed by crown ether-based molecular recognition and disulfide bond connection. J. Polym. Sci. Pol. Chem. 53, 2079–2084 (2015)
Chen, R.F., Knutson, J.R.: Mechanism of fluorescence concentration quenching of carboxyfluorescein in liposomes: energy transfer to nonfluorescent dimers. Anal. Biochem. 172, 61–77 (1988)
Kim, H. N., Lee, M. H., Kim, H. J., Kim, J. S., Yoon, J.: A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 37, 1465–1472 (2008)
Meng, Q., Yu, M., Zhang, H., Ren, J., Huang, D.: Synthesis and application of N-hydroxysuccinimidyl rhodamine B ester as an amine-reactive fluorescent probe. Dyes Pigm. 73, 254–260 (2007)
Kashida, M., Asanuma, H., Komiyama, M.: Insertion of two pyrene moieties into oligodeoxyribonucleotides for the efficient detection of deletion polymorphisms. Chem. Commun. 2768–2770 (2006)
Conroy, E.M., Kim, H., Alger, W.R.: Self-quenching, dimerization, and homo-FRET in hetero-FRET assemblies with quantum dot donors and multiple dye acceptors. J. Phys. Chem. C. 120, 17817–17828 (2016)
Balendiran, G.K., Dabur, R., Fraser, D.: The role of glutathione in cancer. Cell Biochem. Funct. 22, 343–352 (2004)
Kong, F., Liang, Z., Luan, D., Liu, X., Xu, K., Tang, B. A.: Glutathione (GSH)-responsive near-infrared (NIR) theranostic prodrug for cancer therapy and imaging. Anal. Chem. 88, 6450–6456 (2016)
Acknowledgements
The present work is supported in part by MEXT (No. 16K05761-0001), Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hayashida, O., Nishino, K. & Kusano, S. Synthesis of a cysteine-linked cyclophane dimer having two rhodamine moieties and its reduction-responsive degradation as studied by fluorescence spectroscopy. J Incl Phenom Macrocycl Chem 87, 267–274 (2017). https://doi.org/10.1007/s10847-017-0696-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-017-0696-6