Abstract
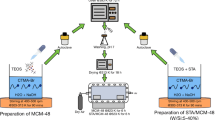
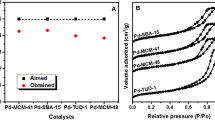
Indium tri-isopropoxide, In(OiPr)3, was immobilized on mesoporous material, MCM-41, and denoted as “In(OiPr)3-MCM-41”. This new heterogeneous catalyst was characterized by XRD, 29Si NMR-, N2 adsorption–desorption isotherms and ICP-OES techniques. The new heterogeneous catalyst, In(OiPr)3-MCM-41, was tested for the capable of catalyzed Meerwein–Ponndorf–Verley (MPV) reduction of unsaturated aldehydes and ketones with low catalyst loadings under mild conditions and showed good to excellent catalytic activities. Also, effect of supporting surfaces, both of SBA-15 and MCM-41, on catalytic activity of In(OiPr)3 were examined. In(OiPr)3-SBA-15 heterogeneous catalyst in comparison with the In(OiPr)3-MCM-41 catalyst, display comparatively higher catalytic activity in the MPV reduction of unsaturated aldehydes and ketones. Also, similiar reaction times and selectivities for the unsaturated alcohols were obtained with the In(OiPr)3-SBA-15 catalyst compared with the In(OiPr)3-MCM-41 catalyst. The reason for the lower activity observed for MCM-41 sample may be due to smaller pore size of the In(OiPr)3-MCM-41 catalyst as compared with In(OiPr)3-SBA-15 catalyst can creat restrict site accessibility for the carbonyl compounds. Eventually, effect of supporting surfaces, SBA-15 and MCM-41, on catalytic activity of In(OiPr)3 insignificant for MPV reduction of unsaturated carbonyl compounds.





Similar content being viewed by others
References
Minambres, J.F., Aramendia, M.A., Marinas, A., Marinas, J.M., Urbano, F.J.: Liquid and gas-phase Meerwein–Ponndorf–Verley reduction of crotonaldehyde on ZrO2 catalysts modified with Al2O3, Ga2O3 and In2O3. J. Mol. Catal. A 338, 121–129 (2011)
Urbano, F.J., Aramendía, M.A., Marinas, A., Marinas, J.M.: An insight into the Meerwein–Ponndorf–Verley reduction of alpha, beta-unsaturated carbonyl compounds: tuning the acid-base properties of modified zirconia catalysts. J. Catal. 268, 79–88 (2009)
Shylesha, S., Mahendra, P.K., Lekh, R.J., Prinson, P.S., Srilakshmic, Ch., Singha, A.P.: Catalytic Meerwein–Ponndorf–Verley reductions over mesoporous silica supports: rational design of hydrophobic mesoporous silica for enhanced stability of aluminum doped mesoporous catalysts. J. Mol. Catal. A 301, 118–126 (2009)
Ooi, T., Miura, T., Itagaki, Y., Ichikawa, H., Maruoka, K.: Catalytic Meerwein–Ponndorf–Verley (MPV) and Oppenauer (OPP) reactions: remarkable acceleration of the hydride transfer by powerful bidentate aluminum alkoxides. Synthesis 18, 279–291 (2002)
Akamanchi, K.G., Varalakshmy, N.R.: Aluminium isopropoxide—TFA, a modified catalyst for highly accelerated meerwein–ponndorf–verley (MPV) reduction. Tetrahedron Lett. 36, 3571–3572 (1995)
Ishii, Y., Nakano, T., Inada, A., Kishigami, Y., Sakurai, K., Ogawa, M.: Meerwein–Ponndorf–Verley type reduction of ketones and Oppenauer type oxidation of alcohols under the influence of bis(cyclopentadienyl)zirconium dihydride. J. Org. Chem. 51, 240–242 (1986)
Knauer, B., Krohn, K.: A reinvestigation of the Meerwein–Ponndorf–Verley reduction a highly efficient variation using zirconium catalysts. Liebigs Ann. 4, 677–683 (1995)
Namy, J.L., Souppe, J., Collin, J., Kagan, H.B.: New preparations of lanthanide alkoxides and their catalytical activity in Meerwein–Ponndorf–Verley–Oppenauer reactions. J. Org. Chem. 49, 2045–2049 (1984)
Lee, J., Ryu, T., Park, S., Lee, P.H.: Indium Tri(isopropoxide)-catalyzed selective Meerwein–Ponndorf–Verley reduction of aliphatic and aromatic aldehydes. J. Org. Chem. 77, 4821–4825 (2012)
Uysal, B., Oksal, B.S.: Comparison of heterogeneous B(OiPr)3-MCM-41 and homogeneous B(OiPr)3, B(OEt)3 catalysts for chemoselective MPV reductions of unsaturated aldehydes and ketones. Appl. Catal. A 435–436, 204–216 (2012)
Uysal, B., Oksal, B.S.: A new method for the chemoselective reduction of aldehydes and ketones using boron tri-isopropoxide, B(OiPr)3: Comparison with boron tri-ethoxide, B(OEt)3. J. Chem. Sci. 123, 681–685 (2011)
Uysal, B., Buyuktas, B.S.: Kinetics of catalytic Meerwein–Ponndorf–Verley reduction of aldehydes and ketones using boron triethoxide. Chem Pap. 64, 123–126 (2010)
Uysal, B., Buyuktas, B.S.: Chemoselective reduction of aldehydes and ketones to alcohols using boron tri-isopropoxide, B(O-i-Pr)3 and boron tri-secondary butoxide, B(O-s-Bu)3 as catalysts. Arkıvoc 14, 134–140 (2007)
Mojtahedi, M.M., Akbarzadeh, E., Sharifi, R., Abaee, M.S.: Lithium bromide as a flexible, mild, and recyclable reagent for solvent-free cannizzaro, tishchenko, and meerwein–ponndorf–verley reactions. Org. Lett. 9, 2791–2793 (2007)
Nishimura, S.: Handbook of heterogeneous catalytic hydrogenation for organic synthesis. Wiley, New York (2001)
Corma, A., Domine, M.E., Valencia, S.: Water-resistant solid Lewis acid catalysts: Meerwein–Ponndorf–Verley and oppenauer reactions catalyzed by tin-beta zeolite. J. Catal. 215, 294–304 (2003)
Boronat, M., Corma, A., Renz, M.: Mechanism of the Meerwein–Ponndorf–Verley–Oppenauer (MPVO) redox equilibrium on Sn– and Zr– beta zeolite catalysts. J. Phys. Chem. B 110, 21168–21174 (2006)
Creyghton, E.J., Ganeshie, S.D., Downing, R.S., Van Bekkum, H.: Stereoselective Meerwein–Ponndorf–Verley and Oppenauer reactions catalysed by zeolite BEA. J. Mol. Catalysis A 115, 457–472 (1997)
Kunkeler, P.J., Zuurdeeg, B.J., van der Waal, J.C., van Bokhoven, J.A., Koningsberger, D.C., Van Bekkum, H.: Zeolite beta: the relationship between calcination procedure, aluminum configuration, and Lewis acidity. J. Catl 180, 234–244 (1998)
Anwander, R., Palm, C.: Meerwein–Ponndorf–Verley reductions mediated by lanthanide-alkoxide-functionalized mesoporous silicates. Stud. Surf. Sci. Catal. 117, 413–420 (1999)
Anwander, R., Palm, C., Gerstberger, G., Groeger, O., Engelhardt, G.: Enhanced catalytic activity of MCM-41-grafted aluminium isopropoxide in MPV reductions. Chem. Commun. 17, 1811–1812 (1998)
Ivanov, V.A., Bachelier, J., Audry, F., Lavalley, J.C.: Study of the Meerwein—Pondorff—Verley reaction between ethanol and acetone on various metal oxides. J. Mol. Catal. 91, 45–59 (1994)
Leyrit, P., Mc Gill, C., Quignard, F., Choplin, A.: A novel heterogeneous molecular catalyst for the Meerwein–Ponndorf–Verley and Oppenauer reactions. J. Mol. Catal. A 112, 395–400 (1996)
Van der Waal, J.C., Creyghton, E.J., Kunkeler, P.J., Tan, K., Van Bekkum, H.: Beta–type zeolites as selective and regenerable catalysts in the Meerwein–Ponndorf–Verley reduction of carbonyl compounds. Top. Catal. 4, 261–268 (1998)
Anwander, R., Runte, O., Eppinger, J., Gerstberger, G., Herdtweck, E., Spiegler, M.: Synthesis and structural characterisation of rare-earth bis(dimethylsilyl)amides and their surface organometallic chemistry on mesoporous MCM-41. J. Chem. Soc. pp. 847–858 (1998)
O’Brien, S., Tudor, J., Barlow, S., Drewitt, M.J., Heyes, S.J., O’Hare, D.: Modification of MCM41 via ring opening of a strained [1] ferrocenophane. Chem. Commun. 6, 641–642 (1997)
Kresege, C.T., Leonowicz, M.E., Roth, W.J., Vartadi, J.C., Beck, J.S.: Ordered mesoporous molecular sieves sythesized by a liquid-crystal template mechanism. Nature 359, 710–712 (1992)
Beck, J.S., Vartuli, J.C., Roth, W.J., Leonowicz, M.E., Kresege, C.T., Schmitt, K.D., Chu, C.T., Olson, D.H., Sheppard, E.W., Mccullen, S.B., Higgius, J.B., Schlenker, J.L.: A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 114, 10834–10843 (1992)
Oberhagemann, U., Jeschke, M., Papp, H.: Synthesis of highly ordered boron-containing B-MCM-41 and pure silica MCM-41. Microporous Mesoporous Mater. 33, 165–172 (1999)
Stevens, W.J.J., Lebeau, K., Mertens, M., van Tendeloo, G., Cool, P., Vansant, E.F.: Investigation of the morphology of the mesoporous SBA-16 and SBA-15 materials. J. Phys. Chem. B 110, 9183–9187 (2006)
Taguchi, A., Schuth, F.: Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 77, 1–45 (2005)
Corma, A.: From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 97, 2373–2419 (1997)
Zhang, F., Yan, Y., Yang, H., Meng, Y., Yu, C., Tu, B., Zhao, D.: Understanding effect of wall structure on the hydrothermal stability of mesostructured silica SBA-15. J. Phys. Chem. B 109, 8723–8732 (2005)
Rahmat, N., Zuhairi, A.A., Rahman Mohamed, A.: A review: mesoporous santa barbara amorphous-15, types, synthesis and its applications towards biorefinery production. Am. J. Appl. Sci. 7, 1579–1586 (2010)
Iglesias, J., Melero, J.A., Morales, G., Moreno, J., Segura, Y., Paniagua, M., Cambra, A., Hernández, B.: Zr-SBA-15 lewis acid catalyst: activity in Meerwein–Ponndorf–Verley reduction. Catalysts 5, 1911–1927 (2015)
Bruyn, M.D., De Vos, D.E., Jacobs, P.A.: Chemoselective hydrogen transfer reduction of unsaturated ketones to allylic alcohols with Solid Zr and Hf catalysts. Adv. Synth. Catal. 344, 1120–1125 (2002)
Uysal, B., Aksu, Y., Oksal, B.S.: Chemoselective reduction of α, β-unsaturated aldehydes and ketones over mesoporous B(OiPr)3–MCM-41 catalyst via MPV reduction process: preparation, characterization and catalytic application. J. Porous Mater. 20, 115–127 (2013)
Uysal, B.: Activity of B(OEt)3-MCM-41 catalyst in the MPV reduction of crotonaldehyde. J. Chem. Sci. 125, 1385–1393 (2013)
Uysal, B., Oksal, B.S.: New heterogeneous B(OEt)3-MCM-41 catalyst for preparation of α, β-unsaturated alcohols. Res. Chem. Intermed. 41, 3893–3911 (2015)
Quignard, F., Graziani, O., Choplin, A.: Group 4 alkyl complexes as precursors of silica anchored molecular catalysts for the reduction of ketones by hydrogen transfer. Appl. Catal. A 182, 29–40 (1999)
Inada, K., Shibagaki, M., Nakanishi, Y., Matsushita, H.: The catalytic reduction of aldehydes and ketones with 2-propanol oversilica-supported zirconium catalyst. Chem. Lett. 22, 1795–1798 (1993)
Zhu, Y., Jaenicke, S., Chuah, G.K.: Supported zirconium propoxide—a versatile heterogeneous catalyst for the Meerwein–Ponndorf–Verley reduction. J. Catal. 218, 396–404 (2003)
Uysal, B., Oksal, B.S.: Catalytic activity of SBA-15-grafted indium tri-isopropoxide in chemoselective MPV reductions. J. Porous Mater. 22, 1053–1064 (2015)
Kinski, I., Gies, H., Marlow, F.: Ordered and disordered pNA molecules in mesoporous MCM-41. Zeolite 19, 375–381 (1997)
Xu, J., Luan, Z., He, H., Zhou, W., Kevan, L.: A reliable synthesis of cubic mesoporous MCM-48 molecular sieve. Chem. Mater. 10, 3690–3698 (1998)
De Graauw, C.F., Peters, J.A., van Bekkum, H., Huskens, J.: Meerwein–Ponndorf–Verley reductions and oppenauer oxidations: an integrated approach. Synthesis 10, 1007–1017 (1994)
Taylor, W.G., Schreck, C.E.: Chiral-phase capillary gas chromatography and mosquito repellent activity of some oxazolidine derivatives of (+)− and (−)−citronellol. J. Pharm. Sci. 74, 534–539 (1985)
Revay, E.E., Kline, D.L., Xue, R.D., Qualls, W.A., Bernier, U.R., Kravchenko, V.D., Ghattas, N., Pstygo, I., Müller, G.C.: Reduction of mosquito biting-pressure: Spatial repellents or mosquito traps? A field comparison of seven commercially available products in Israel. Acta Trop. 127, 63–68 (2013)
Songkro, S., Hayook, N., Jaisawang, J., Maneenuan, D., Chuchome, T., Kaewnopparat, N.: Investigation of inclusion complexes of citronella oil, citronellal and citronellol with [beta]-cyclodextrin for mosquito repellent. J. Incl. Phenom. Macrocycl. Chem. 72, 339–355 (2011)
Song, W., Liu, X.H., Shi, Y.: Citronellol terpenoid inhibits cancer cell proliferation and induces apoptosis in non-small cell lung carcinoma. Lat. Am. J. Pharm. 34, 1652–1657 (2015)
Lopez, J., Valente, J.S., Clacens, J.M., Figueras, F.: Hydrogen transfer reduction of 4-tert-butylcyclohexanone and aldol condensation of benzaldehyde with acetophenone on basic solids. J. Catal. 208, 30–37 (2002)
Samuel, P.P., Shylesh, S., Singh, A.P.: Catalytic properties of tin-containing mesoporous molecular sieves in the selective reduction of carbonyl compounds (Meerwein–Ponndorf–Verley (MPV) reaction). J. Mol. Catal. A 266, 11–20 (2007)
Acknowledgements
The financial support of Scientific and Technical Research Council of Turkey (TUBITAK) under Grant No. 113Z389 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karatas, B.U., Oksal, B.S. & Karatas, E. New In(OiPr)3-MCM-41 heterogeneous catalyst in MPV reductions of unsaturated carbonyl compounds: effect of mesoporous SBA-15 and MCM-41 as supporting surfaces on catalytic activity of In(OiPr)3 . J Incl Phenom Macrocycl Chem 87, 85–94 (2017). https://doi.org/10.1007/s10847-016-0680-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-016-0680-6