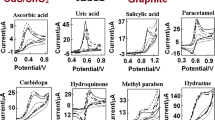
Abstract
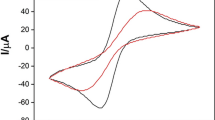
Electrochemical response of a carbon paste electrodes modified by cucurbit[8]uril (Q[8]) has been described. The electrochemical characterization of Q[8]-modified electrode (Q[8]MCPE) by using cyclic voltammetry exhibits the recognition to phenols. For two series of substrates, o-, m-, p-hydroxybenzyl alcohol and o-, m-, p-methoxyphenol, the special response depends on the structures of substrates, the modification with macrocyclic compound Q[8] always favors m-substituted phenols.
Graphical Abstract
The electrochemical response of a cucurbit[8]uril-modified carbon paste electrodes to phenols has been described.







Similar content being viewed by others
References
Ong, W., Kaifer, M.G., Kaifer, A.E.: Cucurbit[7]uril: a very effective host for Viologens and their cation radicals. Org. Lett. 10, 1791 (2002)
Kim, H.J., Heo, J., Jeon, W.S., Lee, E., Kim, J., Sakamoto, S., Yamaguchi, K.: Selective inclusion of a hetero-guest pair in a molecular host: formation of stable charge-transfer complexes in cucurbit[8]uril. Angew. Chem. Int. Ed. 40, 1526 (2001)
Jeon, Y.J., Bhradawaj, P.K., Chio, S.W., Lee, J.W., Kim, K.: Supramolecular amphiphiles: spontaneous formation of vesicles triggered by formation of a charge transfer complex in a host. Angew. Chem. Int. Ed. 41, 4474 (2002)
Alberto, G.C., Pascal, J., Jurriaan, H.: Cucurbit[7]uril self-assembled monolayers studied with force spectroscopy. Langmuir 27, 11508 (2011)
Liu, J.S., Du, X.Z.: Cucurbit[7]uril pseudorotaxanes based on mesoporous silica supports for controlled release. J. Mater. Chem. 20, 3642 (2010)
Tian, F., Cheng, N., Nouvel, N., Geng, J., Scherman, O.A.: Site-selective immobilization of colloids on Au substrates via a noncovalent supramolecular “handcuff”. Langmuir 26, 5323 (2010)
Correia, H.D., Demets, G.J.-F.: Cucurbit[6]uril/PVC-based semipermeable membranes as electrode modifiers for electrochemical investigation of insoluble substrates. Electrochem. Commun. 11, 1928 (2009)
del Pozo, M., Hernández, P., Hernández, L., Quintana, C.: Cucurbit[8]uril host-guest interactions in the development of an electrochemical sensor: characterization and application to tryptophan determination. J. Mater. Chem. 21, 13657 (2011)
Buaki-Sogo, M., del Pozo, M., Hernández, P., García, H., Quintana, C.: Graphene in combination with cucurbit[n]urils as electrode modifiers for electroanalytical biomolecules sensing. Talanta 101, 135 (2012)
Ferancová, A., Korgová, E., Zima, J., Barek, J.: Cyclodextrin modified carbon paste based electrodes as sensors for the determination of carcinogenic polycyclic aromatic amines. Electroanal. 23, 1668 (2002)
Ijeri, V.S., Algarra, M., Martins, A.: Electrocatalytic determination of vitamin C using calixarene modified carbon paste electrodes. Electroanal 24, 2082 (2004)
El-Ries, M.A., Ghany, M.F.A., Hussin, L.A., El-Anwar, F.M., Mohamed, A.M.: Voltammetric behavior of ketoconazole and its determination in cosmetic preparation using a β-cyclodextrin modified glassy carbon electrode. Bull. Facul. of Pharm. Cairo Univ. 51, 49 (2013)
Sakly, N., Souiri, M., Fekih Romdhane, F., Ben Ouada, H., Jaffrezic-Renault, N.: Platinum electrode functionalized with calix [4] arene thin films for impedimetric detection of sodium ions. Mater. Sci. Eng. C 21, 47 (2002)
Asan, A., Isildak, I.: Determination of major phenolic compounds in water by reversed-phase liquid chromatography after pre-column derivatization with benzoyl chloride. J. Chromatogr. A 988, 151 (2003)
Kim, K.R., Kim, H.: Validated gas chromatographic–mass spectrometric analysis of urinary cannabinoids purified with a calcium-hardened β-cyclodextrin polymer. J. Chromatogr. A 886, 87 (2000)
Herberer, T., Stan, H.-J.: Detection of more than 50 substituted phenols as their t-butyldimethylsilyl derivatives using gas chromatography-mass spectrometry. Anal. Chim. Acta 341, 21 (1997)
Nagaraja, P., Vasantha, R.A., Sunitha, K.R.: A sensitive and selective spectrophotometric estimation of catechol derivatives in pharmaceutical preparations. Talanta 55, 1039 (2001)
Nozaki, O., Iwaeda, T., Kato, Y.: Amines for detection of dopamine by generation of hydrogen peroxide and peroxyoxalate chemiluminescence. J. Biolumin. Chemilumin. 11, 309 (1996)
Liu, S.Q., Yu, J.H., Ju, H.X.: Amines for detection of dopamine by generation of hydrogen peroxide and peroxyoxalate chemiluminescence. J. Electroanal. Chem. 540, 61 (2003)
Kozminski, K.D., Gutman, D.A., Davila, V., Sulzer, D., Ewing, A.G.: Voltammetric and pharmacological characterization of dopamine release from single exocytotic events at rat pheochromocytoma (PC12) cells. Anal. Chem. 70, 3123 (1998)
Hu, S.S., Xu, C.L., Wang, G.P.: Voltammetric determination of 4-nitrophenol at a sodium montmorillonite-anthraquinone chemically modified glassy carbon electrode. Talanta 54, 115 (2001)
Fernández, L., Borrás, C., Carrero, H.: Electrochemical behavior of phenol in alkaline media at hydrotalcite-like clay/anionic surfactants/glassy carbon modified electrode. Electrochim. Acta 52, 872 (2006)
Du, H.J., Ye, J.S., Zhang, J.Q.: A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. J. Electroanal. Chem. 650, 209 (2011)
Wang, Y.H., Cong, H., Zhao, F.F., Xue, S.F., Tao, Z., Zhu, Q.J., Wei, G.: Selective catalysis for the oxidation of alcohols to aldehydes in the presence of cucurbit[8]uril. Catal. Commun. 12, 1127 (2011)
Zimmer, H., Lankin, D.C., Horgan, S.W.: Oxidations with potassium nitrosodisulfonate (Fremy’s radical). Teuber reaction. Chem. Rev. 71, 229 (1971)
Magdzia, D., Rodriguez, A.A., Van De Water, R.W., Pettus, T.R.R.: Regioselective oxidation of phenols to o-quinones with o-iodoxybenzoic acid (IBX). Org. Lett. 4, 285 (2002)
Cong, H., Li, Z.J., Wang, Y.H., Tao, Z., Yamato, T., Xue, S.F., Wei, G.: Substituent effect of substrates on cucurbit[8]uril-catalytic oxidationof aryl alcohols. J. Mol. Catal. A: Chem. 374-375, 32 (2013)
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A, Vreven Jr. T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, and Pople J A, 2004 Gaussian 03, Revision C.02 Gaussian, Inc., Wallingford CT
Isaacs, L.: Cucurbit[n]urils: from mechanism to structure and function. Chem. Commun. 6, 619 (2009)
Acknowledgments
We acknowledge the support of National Natural Science Foundation of China (No. 21162003), the International Collaboration Project of Guizhou Province (No. [2011]7003), and the “Chun Hui” Project of the Chinese Ministry of Education (No. Z2014087).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cong, H., Li, ZJ., Geng, QX. et al. Modification of carbon paste electrode with cucurbit[8]uril and its recognition to phenols. J Incl Phenom Macrocycl Chem 81, 493–498 (2015). https://doi.org/10.1007/s10847-015-0480-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-015-0480-4