Abstract
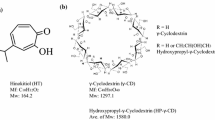
The water solubility of nystatin was found enhanced by forming inclusion complex with gamma-cyclodextrin (γ-CD). Further discovery of a pleased surprise showed that the phase solubility curves of nystatin in β- and γ-CD aqueous solution were AL type, while BS type for α-CD, indicating 1:1 inclusion complexes were formed between β-CD, γ-CD and nystatin, but no inclusion complexes for α-CD, in addition, CDs with much larger ring would be more suitable for forming inclusion complexes with macrolide antibiotics. The aqueous solubility of nystatin in γ-CD solution was investigated increased with γ-CD concentration increasing. At the concentration of 24 g/100 ml for γ-CD aqueous solution, which is near to the saturated solution, water solubility of nystatin was found to be 104 μg/ml, which was 103 folds over original nystatin. Inclusion constants for γ-CD–nystatin complexes were 0.539 l/mmol, which is larger than that of β-CD–nystatin complex (0.375 l/mmol). The inclusion complex of γ-CD with nystatin was prepared and detected by infrared spectrum, results showing that the ester linkage and diene were included in the cavity of CDs, while conjugate arachidonic, carboxyl and amino group were left outside of CDs. Storing experiment showed that forming of the inclusion complexes greatly enhanced the stability of nystatin against light and oxygen.







Similar content being viewed by others
References
Saenger, W.: Crystal packing patterns of cyclodextrin inclusion complexes. J. Incl. Phenom. 2, 445–454 (1984)
Saenger, W., Jacob, J., Gessler, K., Steiner, T., Hoffmann, D., Sanbe, H., Koizumi, K., Smith, S.M., Akaha, T.: Structures of the common cyclodextrins and their larger analogues—beyond the doughnut. Chem. Rev. 98(5), 1787–1802 (1998)
Tafazzoli, M., Ghiasi, M.: Structure and conformation of α-, β- and γ-cyclodextrin in solution: theoretical approaches and experimental validation. Carbohydr. Polym. 78(1), 10–15 (2009)
Bender, M.L., Komiyama, M.: Cyclodextrin Chemistry. Springer, New York (1978)
Koontz, J.L., Marcy, J.E.: Formation of natamycin: cyclodextrin inclusion complexes and their characterization. J. Agric. Food Chem. 51, 7106–7110 (2003)
Koontz, J.L., Marcy, J.E., Barbeau, W.E., Duncan, S.E.: Stability of natamycin and its cyclodextrin inclusion complexes in aqueous solution. J. Agric. Food Chem. 51, 7111–7114 (2003)
Yuan, C., Jin, Z., Xu, X., Zhuang, H., Shen, W.: Preparation and stability of the inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Food Chem. 109, 264–268 (2008)
Szejtli, J.: Past, present and future of cyclodextrin research. Pure Appl. Chem. 76(10), 1825–1845 (2004)
Valle, E.M.M.D.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 6(2), E329–E357 (2005)
Akaike, N., Harata, N.: Nystain perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn. J. Appl. Phys. 44, 433–473 (1994)
Vermerie, N., Malbrunot, C., Azar, M., Arnaud, P.: Stability of nystatin in mouthriness; effect of pH, temperature, concentration and colloidal solver addition, studied using an in vitro antifungal activity. Pharm. World Sci. 19(4), 197–201 (1997)
Shenin, Yu.D., Belakhov, V.V., Araviishii, R.A.: Nystatin: methods of preparation, search for derivatives, and prospects for medicinal use. Khimiko-farmatsevticheskii Zhurnal 27(2), 14–21 (1993)
Higuchi, T., Connors, K.A.: Phase-solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965)
Szejtli, J.: Inclusion of guest molecules, selectivity and molecular recognition by cyclodextrins. In: Szejtli, J., Osa, T. (eds.) Cyclodextrins. Comprehensive Supramolecular Chemistry, vol. 3, pp. 189–203. Elsevier Science, Amsterdam (1996)
Seridi, L., Boufelfel, A.: Simulations of docking C60 in β-cyclodextrin. J. Mol. Liq. 162, 69–77 (2011)
Acknowledgments
This project was supported by National Natural Science Foundation (Grant No. 31230057). The authors thank financial support by the Fundamental Research Funds for the Central Universities (JUSRP111A30 and JUSRP11122), and by Science and Technology of the Twelfth Five-Year Plan (2012BAD37B02, 2012BAD37B03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Jin, Z. & Xu, X. Gamma-cyclodextrin on enhancement of water solubility and store stability of nystatin. J Incl Phenom Macrocycl Chem 78, 145–150 (2014). https://doi.org/10.1007/s10847-012-0281-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0281-y