Abstract
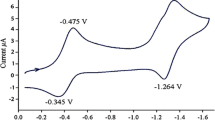
Four nano-baskets of calixarene including cone 25,27-di(carboxymethoxy)calix[4]arene-crown-5, 1,3-alternate 25,27-di[carboxymethoxy]-calix[4]arene-crown-5, cone 25,27-bis[carboxymethoxy]calix[4]arene-crown-6 and 1,3-alternate 25,27-di[carboxymethoxy]-calix[4]arene-crown-6 were synthesized and their binding abilities towards alkali and alkaline earth metals as well as some lanthanides were studied using differential pulse voltammetry. The novelty of this study was investigation of those macrocyclic complexes by voltammetric behaviors of two acidic moieties in each scaffold during complexation of crown ether ring. Their voltammetric behaviors were closely related to the complex formation by entrapment of cation into crown ether cavity and ion–dipole interaction between cation and acidic moieties in calixcrowns. The results revealed the selective changes in voltammetric behavior of synthesized scaffolds toward the cations. Moreover, the position of crown ether in 1,3-alternate instead of cone enhanced the domain of binding ability to more cations. Furthermore, it was shown that those carboxylic acid moieties, which were far from the crown ether ring in the 1,3-alternate, did not affected by encapsulated cations in the coordination space of crown ether and showed no voltammetric behavior.



Similar content being viewed by others
References
Mokhtari, B., Pourabdollah, K., Dalali, N.: Analytical applications of calixarenes from 2005 up-to-date. J. Incl. Phenom. Macrocycl. Chem. 69(1–2), 1–55 (2011)
Mokhtari, B., Pourabdollah, K., Dalali, N.: Molecule and ion recognition of nano-baskets of calixarenes since 2005. J. Coord. Chem. 64(5), 743–794 (2011)
Mokhtari, B., Pourabdollah, K., Dallali, N.: A review of calixarene applications in nuclear industries. J. Radioanal. Nucl. Chem. 287(3), 921–934 (2011)
Mokhtari, B., Pourabdollah, K., Dalali, N.: Applications of nano-baskets of calixarenes in chromatography. Chromatographia 73(9–10), 829–847 (2011)
Mokhtari, B., Pourabdollah, K.: Advances in binding ability and extractive applications of nano-baskets of calixarene. Asian J. Chem. 23(11), 4714–4734 (2011)
Salorinne, K., Nissinen, M.: Calixcrowns: synthesis and properties. J. Incl. Phenom. Macrocycl. Chem. 61(1–2), 11–27 (2008)
Kim, J.S., Vicens, J.: Progress of calixcrowns chemistry. J. Incl. Phenom. Macrocycl. Chem. 63(1–2), 189–193 (2009)
Lamare, V., Dozol, J.F., Ugozzoli, F., Casnati, A., Ungaro, R.: X-ray crystal structures and molecular modelling studies of calix[4]dibenzocrowns-6 and their alkali metal cation complexes. Eur. J. Org. Chem. 1998(8), 1559–1568 (1998)
Mokhtari, B., Pourabdollah, K.: Competitive solvent extraction of alkaline earth metals by ionizable nano-baskets of calixarene. Supramol. Chem. 23, 696–702 (2011)
Mokhtari, B., Pourabdollah, K.: Medical applications of nano-baskets. J. Coord. Chem. 64, 3189–3204 (2011)
Mokhtari, B., Pourabdollah, K.: Effect of crown size and upper moieties in nano-baskets of diacid calix[4]arene-1,2-crowns-3,4,5,6 on the extraction of s-block metals. J. Coord. Chem. 64, 3081–3091 (2011)
Mokhtari, B., Pourabdollah, K.: Solvent extraction of alkali metals by di-ionizable nano-baskets. J. Coord. Chem. 64, 4029–4053 (2011)
Mokhtari, B., Pourabdollah, K.: Investigation of ionizable nano-baskets of calix[4]-1,2-crown-3 by differential pulse voltammetry. J. Coord. Chem. 64, 4079–4087 (2011)
Mokhtari, B., Pourabdollah, K.: Structure optimization of di-ionizable calixarene nano-baskets for competitive solvent extraction of alkali and alkaline earth metals. Bull. Korean Chem. Soc. 32, 3855–3860 (2011)
Mokhtari, B., Pourabdollah, K.: Effect of crown ring size and upper moiety on the extraction of s-block metals by ionizable calixcrown nano-baskets. Bull. Korean Chem. Soc. 32, 3979–3990 (2011)
Mokhtari, B., Pourabdollah, K.: Inclusion desalination of alkali metal cations by emulsion liquid membranes and nano-baskets of p-tert-calix[4]arene bearing di-[n-(x)sulfonyl carboxamide] and di-(1-propoxy) in para-cone conformation. Desalination 292, 1–8 (2012)
Mokhtari, B., Pourabdollah, K.: Determination of salbutamol in livestock by nano-mediated bonded-phases: nano-baskets of calixarene in partial-cone conformation. J. Chilean Chem. Soc. 58, 827–831 (2012)
Mokhtari, B., Pourabdollah, K.: Applications of calixarene nano-baskets in pharmacology. J. Incl. Phenom. Macrocycl. Chem. 73, 1–15 (2012). doi:10.1007s10847-011-0062-z
Mokhtari, B., Pourabdollah, K.: Applications of nano-baskets in drug development: high solubility and low toxicity. Drug Chem. Toxicol. (2012). doi:10.3109/01480545.2011.653490
Mokhtari, B., Pourabdollah, K.: Binding and extraction of alkali and alkaline earth metals by nano-baskets of calix[4]arene-1,2-crown-3 conformers. J. Incl. Phenom. Macrocycl. Chem. 73, 269–277 (2012). doi:10.1007/s10847-011-0052-1
Mokhtari, B., Pourabdollah, K.: Binding mechanisms of nano-baskets towards alkali metals. Isothermal titration calorimetric study. J. Therm. Anal. Calorim. (2012). doi:10.1007/s10973-011-2014-7
Mokhtari, B., Pourabdollah, K.: Extraction of vanadyl porphyrins in crude oil by inclusion dispersive liquid–liquid microextraction and nano-baskets of calixarene. J. Incl. Phenom. Macrocycl. Chem. (2012). doi:10.1007/s10847-011-0099-z
Mokhtari, B., Pourabdollah, K.: Binding survey of ionizable calix[4]-1,2-crown-3 nano-baskets by differential pulse voltammetry. Electroanalysis 24(2), 219–223 (2012)
Mokhtari, B., Pourabdollah, K.: Binding study of ionizable calix[4]-1,3-crowns-5,6 nano-baskets by differential pulse voltammetry. J. Electrochem. Soc. 159(3), K61–K65 (2012)
Mokhtari, B., Pourabdollah, K.: Dispersive enhancement in liquid–liquid microextraction by dual supramolecular role of nano-baskets. Supramol. Chem. 24(4), 255–263 (2012)
Mokhtari, B., Pourabdollah, K.: Chromatographic separation of clenbuterol by bonded-phases bearing nano-baskets of p-tert-calix[4]-1,2-crown-3; -crown-4; -crown-5; and -crown-6. J. Sci. Food Agric. (2012). doi:10.1002/jsfa.5688
Mokhtari, B., Pourabdollah, K.: Inclusion extraction of alkali metals by emulsion liquid membranes and nano-baskets of p-tert-calix[4]arene bearing di-[N-(X)sulfonyl carboxamide] and di-(1-propoxy) in ortho-cone conformation. Bull. Korean Chem. Soc. 33(5), 1509–1516 (2012)
Mokhtari, B., Pourabdollah, K.: Extraction of s-block metals by nano-baskets of calix[4]crown-3. Can. J. Chem. 90(6), 560–566 (2012)
Mokhtari, B., Pourabdollah, K.: Emulsion liquid membranes for effective inclusion extraction of alkali metals by nano-baskets of calixarene. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. (2012). doi:10.1080/15533174.2012.654879
Zheng, H., Yan, Z., Dong, H., Ye, B.: Simultaneous determination of lead and cadmium at a glassy carbon electrode modified with Langmuir–Blodgett film of p-tert-butylthiacalix[4]arene. Sens. Actuators B 120(2), 603–609 (2007)
Dong, H., Zheng, H., Lin, L., Ye, B.: Determination of thallium and cadmium on a chemically modified electrode with Langmuir–Blodgett film of p-allylcalix[4]arene. Sens. Actuators B 115(1), 303–308 (2006)
Raoof, J.B., Ojani, R., Alinezhad, A., Rezaie, S.Z.: Differential pulse anodic stripping voltammetry of silver(I) using p-isopropylcalix[6]arene modified carbon paste electrode. Monatshefte Chem. 141(3), 279–284 (2010)
Wang, L., Zhao, B.T., Ye, B.X.: Electrochemical properties of electrode modified with Langmuir–Blodgett film of p-tertbutylcalix[4]arene derivatives and its application in determining of silver. Electroanalysis 19(9), 923–927 (2007)
Dong, H., Lin, L., Zheng, H., Zhao, G., Ye, B.: Electrode modified with Langmuir–Blodgett (LB) film of calixarenes for preconcentration and stripping analysis of Hg(II). Electroanalysis 18(12), 1202–1207 (2006)
Wang, F., Liu, J., Wu, Y.J., Gao, Y.M., Huang, X.F.: Anodic stripping voltammetric determination of mercury(II) in water using a 4-tert-butyl-1-(ethoxycarbonylmethoxy)thiacalix[4]arene modified glassy carbon electrode. J. Chin. Chem. Soc. 56, 778–784 (2009)
Canpolat, E.C., Sar, E., Coskun, N.Y., Cankurtaran, H.: Determination of trace amounts of copper in tap water samples with a calix[4]arene modified carbon paste electrode by differential pulse anodic stripping voltammetry. Electroanalysis 19(10), 1109–1115 (2007)
Shamsipur, M., Miranbeigi, A.A., Teymouri, M., Rasoolipour, S., Asfari, Z.: Highly sensitive and selective poly(vinyl chloride)-membrane potentiometric sensors based on a calix[4]arene derivative for 2-furaldehyde. Anal. Chem. 81(16), 6789–6796 (2009)
Snejdarkova, M., Poturnayova, A., Rybar, P., Lhotak, P., Him, M., Flidrova, K., Hianik, T.: High sensitive calixarene-based sensor for detection dopamine by electrochemical and acoustic methods. Bioelectrochemistry 80(1), 55–61 (2010)
Vaze, V.D., Srivastava, A.K.: Electrochemical behavior of folic acid at calixarene based chemically modified electrodes and its determination by adsorptive stripping voltammetry. Electrochim. Acta 53, 1713–1721 (2007)
Zhang, H.L., Liu, Y., Lai, G.S., Yu, A.M., Huang, Y.M., Jin, C.M.: Calix[4]arene crown-4 ether modified glassy carbon electrode for electrochemical determination of norepinephrine. Analyst 134, 2141–2146 (2009)
Lai, G.S., Zhang, H.L., Jin, C.M.: Electrocatalysis and voltammetric determination of dopamine at a calix[4]arene crown-4 ether modified glassy carbon electrode. Electroanalysis 19(4), 496–501 (2007)
Liu, H., Zhao, G., Wen, L., Ye, B.: Simultaneous voltammetric determination of epinephrine and serotonin at a p-tetra-butyl calix[6]arene-l-histidine chemically modified electrode. J. Anal. Chem. 61(11), 1104–1107 (2006)
Amiri, A., Choi, E.Y., Kim, H.J.: Development and molecular recognition of calixcrownchip as an electrochemical ALT immunosensor. J. Incl. Phenom. Macrocycl. Chem. 66(1–2), 185–194 (2010)
Kotkar, R.M., Srivastava, A.K.: Electrochemical behavior of nicotinamide using carbon paste electrode modified with macrocyclic compounds. J. Incl. Phenom. Macrocycl. Chem. 60(3–4), 271–279 (2008)
Sosovska, O., Korpan, Y., Vocanson, F., Jaffrezic-Renault, N.: Conductometric chemosensors based on calixarenes for determination of amines and amino acids. Sens. Lett. 7(5), 989–994 (2009)
Filenko, D., Gotszalk, T., Kazantseva, Z., Rabinovych, O., Koshets, I., Shirshov, Yu., Kalchenko, V., Rangelow, I.W.: Chemical gas sensors based on calixarene-coated discontinuous gold films. Sens. Actuators B 111–112, 264–270 (2005)
Ijeri, V., Vocanson, F., Martelet, C., Jaffrezic-Renault, N.: Capacitive sensing of amino acids using caliraxene-coated silicon transducers. Electroanalysis 19(4), 510–514 (2007)
Nikolelis, D.P., Raftopoulou, G., Psaroudakis, N., Nikoleli, G.P.: Development of an electrochemical biosensor for the rapid detection of carbofuran based on air stable lipid films with incorporated calix[4]arene phosphoryl receptor. Electroanalysis 20(14), 1574–1580 (2008)
Hassen, W.M., Martelet, C., Davis, F., Higson, S.P.J., Abdelghani, A., Helali, S., Jaffrezic-Renault, N.: Calix[4]arene based molecules for amino-acid detection. Sens. Actuators B 124(1), 38–45 (2007)
Mokhtari, B., Pourabdollah, K.: Voltammetric study of nano-baskets of calix[4]-1,3-crowns-5, crowns-6 complexes. Electrochim. Acta 76, 363–367 (2012).
Mokhtari, B., Pourabdollah, K.: Nano-baskets in emulsion liquid membranes for selective extraction of alkali metals. J. Chinese Chem. Soc. (2012). doi:10.1002/jccs.201100737
Mokhtari, B., Pourabdollah, K.: Preparation and characterization of bonded-phases of nano-baskets bearing sulfonyl-carboxamide. J. Liq. Chromatogr. Relat. Tech. (2012). doi:10.1080/10826076.2011.643524
Acknowledgments
This work was supported by Islamic Azad University (Shahreza branch) and Iran Nanotechnology Initiative Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mokhtari, B., Pourabdollah, K. Electrochemical investigation of nano-baskets of calix[4]-1,3-crowns-5,6 complexes . J Incl Phenom Macrocycl Chem 76, 385–390 (2013). https://doi.org/10.1007/s10847-012-0210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0210-0