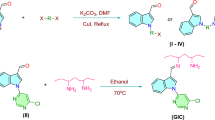
Abstract
The inclusion complex of isatoic anhydride with β-cyclodextrin was formed as a result of intermolecular interaction between isatoic anhydride with β-CD. The inclusion complex was confirmed by IR spectroscopy, X-ray diffraction and DSC studies. From application of complex, herein we have described a simple and efficient protocol for synthesis of 2, 3-dihydroquinazoline-4(1H)-one derivatives by one pot condensation of isatoic anhydride, ammonium acetate or amine and aldehyde using β-CD as a supramolecular catalyst in aqueous media.






Similar content being viewed by others
References
Breslowong, R., Dong, S.D.: Biomimetic reactions catalyzed by cyclodextrins and their derivatives. Chem. Rev. 98, 1997–2011 (1998)
Desper, J.M., Breslow, R.: Catalysis of intramolecular aldol condensation by imidazole bearing cyclodextrin. J. Am. Chem. Soc. 116, 12081–12082 (1994)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Bhosale, S.V., Bhosale, S.V.: Beta cyclodextrin as a catalyst in organic synthesis. Min. Rev. Org. Chem. 4, 143–157 (2007)
Coppola, G.M., Schuster, H.F.: Application of isatoic anhydride chemistry to the synthesis of racemic paraensine. J. Heterocyl. Chem. 21, 1409–1410 (1984)
Clark, R.H., Wagner, E.C.: Isatoic anhydride I reaction with primary and secondary amines and with some amides. J. Org. Chem. 9, 55–67 (1944)
Kappe, T., Stadlbour, W.: Isatoic anhydride and their uses in heterocyclic synthesis. Adv. Heterocly. Chem. 28, 127–182 (1981)
Na, Y.H., Hong, S.H., Lee, J.H., Park, W.K., Baek, D.J., Koh, H.Y., Cho, Y.S., Choo, H., Pae, A.N.: Novel quinazolinone derivatives as 5-HT7 receptor ligands. Bioorg. Med. Chem. 16, 2570–2578 (2008)
Wolfe, J.F., Rathman, T.L., Sleevi, M.C., Campbell, J.A., Greenwood, T.D.: Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones. J. Med. Chem. 33, 161–166 (1990)
Mhaske, S.B., Argade, P.: The chemistry of recently isolated naturally occurring quinazoline alkaloids. Tetrahedron 62, 9787–9826 (2006)
Kametani, T.: Loc, CV, Higa, T., Koizumi, M., Ihara, M., Fukumoto, K.J.: Iminoketene cycloaddition. 2. Total syntheses of arborine, glycosminine, and rutecarpine by condensation of iminoketene with amides. J. Am. Chem. Soc. 99, 2306–2309 (1977)
Mason, J.J., Bergman, J.: Total synthesis of luotonin A and 14-substituted analogues. Org. Biomol. Chem. 5, 2486–2490 (2007)
Liu, J.-F., Ye, P., Sprague, K., Sarget, K., Yohannes, D., Baldino, C.M., Wilson, C.J., Ng, S.C.: Novel One-pot total syntheses of deoxyvasicinone, mackinazolinone, isaindigotone, and their derivatives promoted by microwave irradiation. Org. Lett. 5, 3363–3366 (2005)
Chinigo, G.M., Paige, M., Grindrod, S., Hamel, E., Dakshanamurthy, S., Chruszcz, M., Minor, W., Brown, M.L.: Asymmetric synthesis of 2,3-dihydro-2-arylquinazolin-4-ones: methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity. J. Med. Chem. 51, 4620–4631 (2008)
Narsimhulu, M., Lee, Y.R.: Ethylenediamine diacetate-catalyzed three-component reaction for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones and their spirooxindole derivatives. Tetrahedron 67, 9627–9634 (2011)
Salehi, P., Dabiri, M., Zolfigol, M.A., Baghban-zadeh, M.: A novel method for the one-pot three-component synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Syn lett 7, 1155–1157 (2005)
Chen, J., Su, W., Wu, H., Liu, M., Jin, C.: Eco-friendly synthesis of 2,3-dihydroquinazolin-4(1H)-ones in ionic liquids or ionic liquid–water without additional catalyst. Green Chem. 9, 972–975 (2007)
Shi, D., Rong, L., Wang, J., Zhung, Q., Wang, X., Hu, H.: Synthesis of quinazoline-4(3H)-ones and 1,2-dihydroquinazolin-4(3H)-ones with the aid of a low-valent titanium reagent. Tetrahedron Lett. 44, 3199–3201 (2003)
Doheri, M., Salehi, P., Otokesh, S., Baghbanzadeh, M., Kozehgary, G., Mohammadi, A.A.: Efficient synthesis of mono- and disubstituted 2,3-dihydroquinazolin-4(1H)-ones using KAl(SO4)2·12H2O as a reusable catalyst in water and ethanol. Tetrahedron Lett. 46, 6123–6126 (2005)
Chen, J., Wu, D., He, F., Liu, M., Wu, H., Su, W.: Gallium(III) triflate-catalyzed one-pot selective synthesis of 2,3-dihydroquinazolin-4(1H)-ones and quinazoline-4(3H)-ones. Tetrahedron Lett. 49, 3814–3818 (2008)
Dabiri, M., Salehi, P., Baghbanzadeh, M.: Ionic Liquid Promoted Eco-friendly and Efficient Synthesis of 2,3-Dihydroquinazolin-4(1H)-ones. Monatsh. Chem. 138, 1191–1194 (2007)
Salehi, P., Dabiri, M., Baghbanzadeh, M., Bahramnejad, M.: One-Pot, Three-component synthesis of 2, 3-dihydro-4(1H)-quinazolinones by Montmorillonite K-10 as an efficient and reusable catalyst. Synth. Commun. 36, 2287–2292 (2006)
Shaterian, H.R., Oveisi, A.R., Honarmand, M.: synthesis of 2, 3-dihydroquinazoline-4(1H)-ones. Synth. Commun. 40, 1231–1242 (2010)
Shaabani, A., Maleki, A., Mofakham, H.: Click Reaction: highly efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Synth. Commun. 38, 3751–3759 (2008)
Abdel-Jalil, R.J., Voelter, W., Saeed, M.: A novel method for synthesis of 4(3H)- quinazolinones. Tetrahedron Lett. 45, 3475–3476 (2004)
Cheng, X., Vellalath, S., Goddard, R., List, B.: Direct catalytic asymmetric synthesis of cyclic aminals from aldehydes. J. Am. Chem. Soc. 130, 15786–15787 (2008)
Rueping, M., Antonchick, A.P., Sugiono, E., Grenader, K.: Asymmetric brønsted acid catalysis: catalytic enantioselective synthesis of highly biologically active dihydroquinazolinones. Angew. Chem. Int. Ed. 48, 908–910 (2009)
Dabiri, M., Salehi, P., Bahramanejad, M., Alizadeh, M.: A practical and versatile approach toward a one-pot synthesis of 2,3-disubstituted 4(3H)-quinazolinones. Monatsch Chem. 141, 877–881 (2010)
Zhang, Z.H., Lu, H.Y., Yang, S.H., Gao, J.W.: Synthesis of 2, 3-dihydroquinazolin-4(1H)-ones by three-component coupling of isatoic anhydride, amines, and aldehydes catalyzed by magnetic Fe3O4 nanoparticles in water. J. Comb. Chem. 12, 643–646 (2010)
Teuri, A.S., Bolouk, S.: One-pot, three-component synthesis of 2,3-dihydroquinazolin- 4(1H)-ones using p-toluenesulfonic acid–paraformaldehyde copolymer as an efficient and reusable catalyst. Monatsch Chem. 141, 1113–1115 (2010)
Niknam, K., Jafoopour, N., Niknam, E.: Silica-binded N-propylsulfamic acid as a recyclable catalyst for synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Chin. Chem. Lett. 22, 69–72 (2011)
Li, C.J., Chan, T.H.: Organic reactions in aqueous media. Wiley, New York (2007)
Patil, D.R., Dalal, D.S.: One-pot, solvent free synthesis of Hantzsch 1, 4-dihydropyridines using β-cyclodextrin as a supramolecular catalyst. Lett. Org. Chem. 8, 477–483 (2011)
Kumar, A., Tripathi, V.D., Kumar, P.: β-Cyclodextrin catalysed synthesis of tryptanthrin in water. Green Chem. 13, 51–54 (2011)
Kemelbekov, U., Luo, Y., Orynbekova, Z., Rustembekov, Z., Haag, R., Saenger, W., Pralivey, K.: IR, UV and NMR studies of β-cyclodextrin inclusion complexes of kazcaine and prosidol bases. J. Incl. Phenom. Macrocycl. Chem. 69, 181–190 (2011)
Choi, S.H., Kim, S.Y., Ryoo, J.J., Park, J.Y., Lee, K.P.: FT-Raman and FTIR spectra of the nonsteroidal antiinflammatory drug ketoprofen included in cyclodextrin. Anal. Sci. 17, 1785–1788 (2001)
Saenger, W.: Cyclodextrin inclusion compounds in research and industry. Angew. Chem. Intl. Ed. Engl. 19, 344–362 (1980)
Acknowledgments
The authors are thankful to Department of Science and Technology and University Grants Commission, New Delhi, India for financial support of this work. We are also thankful to Dr. K. J. Patil, Emeritus Professor, School of Chemical Sciences for useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, D.R., Ingole, P.G., Singh, K. et al. Inclusion complex of Isatoic anhydride with β-cyclodextrin and supramolecular one-pot synthesis of 2, 3-dihydroquinazolin-4(1H)-ones in aqueous media. J Incl Phenom Macrocycl Chem 76, 327–332 (2013). https://doi.org/10.1007/s10847-012-0203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0203-z