Abstract
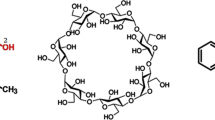
This paper reports a molecular modeling study of complex formation and aggregation behavior of a supramolecular system comprising three different moieties forming two distinct molecules. One molecule is a phenol derivative of porphyrin conjugated to a macrocyclic oligosaccharide, β-cyclodextrin (β-CD), and the other is 1-adamantanol (ADM). The inclusion complex of the latter molecule with the porphyrin–β-cyclodextrin (β-CD) conjugate, and the dimeric aggregates of the conjugate both in the presence and in the absence of the guest are investigated through molecular mechanics and molecular dynamics methods in vacuo, since the systems are scarcely soluble in polar solvents. In this way, we can find the most likely geometry of the complexes or aggregates and characterize the competitive inclusion behavior of ADM and of a porphyrin phenol within the β-CD cavity in terms of the various energy contributions stabilizing the resulting aggregates and/or inclusion complexes.








Similar content being viewed by others
References
Rekharsky, M.V., Inoue, Y.: Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1917 (1998)
Lipkowitz, K.B.: Applications of computational chemistry to the study of cyclodextrins. Chem. Rev. 98, 1829–1873 (1998)
Nagaraju, M., Sastry, G.N.: Theoretical studies on inclusion complexes of cyclodextrins. J. Phys. Chem. A 113, 9533–9542 (2009)
Kuwabara, T., Takamura, M., Matsushita, A., Ikeda, H., Nakamura, A., Ueno, A., Toda, F.: Phenolphthalein-modified β-cyclodextrin as a molecule-responsive colorless-to-color change indicator. J. Org. Chem. 63, 8729–8735 (1998)
González-Álvarez, M.J., Balbuena, P., Mellet, C.O., García Fernández, J.M., Mendicuti, F.: Study of the conformational and self-aggregation properties of 2I,3I-O-(o-Xylylene)-per-O-Me-α- and β-cyclodextrins by fluorescence and molecular modeling. J. Phys. Chem. B 112, 13717–13729 (2008)
Carmona, T., González-Álvarez, M.J., Mendicuti, F., Tagliapietra, S., Martina, K., Cravotto, G.: Structure and self-aggregation of mono- and bis(cyclodextrin) derivatives in aqueous media: fluorescence, induced circular dichroism, and molecular dynamics. J. Phys. Chem. C 114, 22431–22440 (2010)
Puglisi, A., Purrello, R., Rizzarelli, E., Sortino, S., Vecchio, G.: Spectroscopic and self-association behavior of a porphyrin–β-cyclodextrin conjugate. New J. Chem. 31, 1499–1506 (2007)
Fathalla, M., Neuberger, A., Li, S.-C., Schmehl, R., Diebold, U., Jayawickramarajah, J.: Straightforward self-assembly of porphyrin nanowires in water: harnessing adamantane/β-cyclodextrin interactions. J. Am. Chem. Soc. 132, 9966–9967 (2010)
Králová, J., Kejík, Z., Bříza, T., Poučková, P., Král, A., Martásek, P., Král, V.: Porphyrin–cyclodextrin conjugates as a nanosystem for versatile drug delivery and multimodal cancer therapy. J. Med. Chem. 53, 128–138 (2010)
Wang, K.-R., Guo, D.-S., Jiang, B.-P., Liu, Y.: Excitonic coupling interactions in the self-assembly of perylene-bridged bis(β-cyclodextrin)s and porphyrin. Chem. Commun. 48, 3644–3646 (2012)
Raffaini, G., Ganazzoli, F., Malpezzi, L., Fuganti, C., Fronza, G., Panzeri, W., Mele, A.: Validating a strategy for molecular dynamics simulations of cyclodextrin inclusion complexes through single-crystal X-ray and NMR experimental data: a case study. J. Phys. Chem. B 113, 9110–9122 (2009)
Raffaini, G., Ganazzoli, F.: Molecular dynamics study of host–guest interactions in cyclodextrins: methodology and data analysis for a comparison with solution data and the solid state structure. J. Incl. Phenom. Macrocyclic Chem. 57, 683–688 (2007)
Raffaini, G., Ganazzoli, F.: A molecular dynamics study of the inclusion complexes of C60 with some cyclodextrins. J. Phys. Chem. B 114, 7133–7139 (2010)
Hoeben, F.J.M., Jonkheijm, P., Meijer, E.W., Schenning, A.P.H.J.: About supramolecular assemblies of π-conjugated systems. Chem. Rev. 105, 1491–1546 (2005)
Accelrys Inc.: InsightII 2000. San Diego. http://www.accelrys.com
Dauber-Osguthorpe, P., Roberts, V.A., Osguthorpe, D.J., Wolff, J., Genest, M., Hagler, A.T.: Structure and energetics of ligand binding to proteins: escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins Struct. Funct. Genet. 4, 31–47 (1988)
Asensio, J.L., Martin-Pastor, M., Jimenez-Barbero, J.: The use of CVFF and CFF91 force fields in conformational analysis of carbohydrate molecules. Comparison with AMBER molecular mechanics and dynamics calculations for methyl α-lactoside. Int. J. Biol. Macromol. 17, 137–148 (1995)
Sueishi, Y., Miyakawa, T.: Complexation of phenols with β- and γ-cyclodextrins: determination of the association constants by using the isomerization of spiropyran. J. Phys. Org. Chem. 12, 541–546 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raffaini, G., Ganazzoli, F. A molecular modeling study of complex formation and self-aggregation behavior of a porphyrin–β-cyclodextrin conjugate. J Incl Phenom Macrocycl Chem 76, 213–221 (2013). https://doi.org/10.1007/s10847-012-0193-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0193-x