Abstract
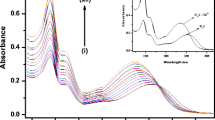
A hetero-bimetallic complex of type [Cu(bpy)(NCS)(μ-NCS)Ru(bpy)2Cl]·PF6 is synthesized and characterized using spectroscopic studies, elemental analyses and ESI-MS data. Owing to the presence of sulphur donor ligand in the complex, its affinity towards soft metal ions is monitored. It detects selectively Hg2+ and Ag+ ions over a number of environmentally relevant cations including Mn2+, Pb2+, Zn2+, Cd2+, Ni2+, Cu2+, and Co2+. The naked eye response is more pronounced for Hg2+ as compared to Ag+ ions. The detection limit for Hg2+ ions is 1.0 ppm while for Ag+ ions are 10 ppm. However, fluorescence response is exclusive to Ag+ ions. The complex shows a tenfold enhancement in the fluorescence intensity upon addition of Ag+ ions while no change in emission intensity is observed upon addition of other cations including Hg2+. Competitive binding experiments using fluorescence spectrometry reveal that the complex binds Ag+ selectively even in presence of tenfold excess of other cations exclusive of Hg2+ ions. The emission from the complex obtained by addition of Ag+ ions is quenched upon addition of Hg2+ ions, displaying fluorescence on–off behavior.
Graphical Abstract











Similar content being viewed by others
References
Nolan, E.M., Lippard, S.J.: Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 108, 3443 (2008)
EPA: Mercury update: impact of fish advisories. EPA fact sheet EPA-823-F-01-011. EPA, Office of Water, Washington D.C. (2001)
Boening, D.W.: Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40, 1335 (2000)
Harris, H.H., Pickering, I.J., George, G.N.: The chemical form of mercury in fish. Science 301, 1203 (2003)
Yoon, S., Albers, A.E., Wong, A.P., Chang, C.J.: Screening mercury levels in fish with a selective fluorescent chemosensor. J. Am. Chem. Soc. 127, 16030 (2005)
Leray, I., Valeur, B.: Calixarene-based fluorescent molecular sensors for toxic metals. Eur. J. Inorg. Chem. 24, 3525 (2009)
Caballero, A., Martınez, R., Lloveras, V., Ratera, I., Vidal-Gancedo, J., Wurst, K., Tarraga, A., Molina, P., Veciana, J.: Highly selective chromogenic and redox or fluorescent sensors of Hg2+ in aqueous environment based on 1,4-disubstituted azines. J. Am. Chem. Soc. 127, 15666 (2005)
Hennrich, G., Walther, W., Resch-Genger, U., Sonnenschein, H.: Cu(II)- and Hg(II)-induced modulation of the fluorescence behavior of a redox-active sensor molecule. Inorg. Chem. 40, 641 (2001)
Shunmugam, R., Gabriel, G.J., Smith, C.E., Aamer, K.A., Tew, G.N.: A highly selective colorimetric aqueous sensor for mercury. Chem. Eur. J. 14, 3904 (2008)
Yang, Y.K., Yook, K.J., Tae, J.: A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J. Am. Chem. Soc. 127, 16760 (2005)
Ou, S., Lin, Z., Duan, C., Zhang, H., Bai, Z.: A sugar-quinoline fluorescent chemosensor for selective detection of Hg2+ ion in natural water. Chem. Commun. 42, 4392 (2006)
Voutsadaki, S., Tsikalas, G.K., Klontzas, E., Froudakis, G.E., Katerinopoulos, H.E.: A “turn-on” coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media. Chem. Commun. 46, 3292 (2010)
Valeur, B., Leray, I.: Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 205, 3 (2000)
Prodi, L., Bolletta, F., Montalti, M., Zaccheroni, N.: Luminescent chemosensors for transition metal ions. Coord. Chem. Rev. 205, 59 (2000)
Deo, S., Godwin, H.A.: A selective, ratiometric fluorescent sensor for Pb2+. J. Am. Chem. Soc. 122, 174 (2000)
Yang, R.-H., Chan, W.-H., Lee, A.W.M., Xia, P.-F., Zhang, H.-K., Li, K.: A ratiometric fluorescent sensor for Ag+ with high selectivity and sensitivity. J. Am. Chem. Soc. 125, 2884 (2003)
Lin, Z.H., Ou, S.J., Duan, C.Y., Zhang, B.G., Bai, Z.P.: Naked-eye detection of fluoride ion in water: a remarkably selective easy-to-prepare test paper. Chem. Commun. 6, 624 (2006)
Dixit, N., Mishra, L., Mustafi, S.M., Chary, K.V.R., Houjou, H.: Synthesis of a ruthenium(II) bipyridyl complex coordinated by a functionalized Schiff base ligand: characterization, spectroscopic and isothermal titration calorimetry measurements of M2+ binding and sensing (M2+=Ca2+, Mg2+). Spectrochim. Acta A 73, 29 (2009)
Shi, Y., Toms, B.B., Dixit, N., Kumari, N., Mishra, L., Goodisman, J., Dabrowiak, J.C.: Cytotoxicity of Cu(II) and Zn(II) 2,2′-bipyridyl complexes: dependence of IC50 on recovery time. Chem. Res. Toxicol. 23, 1417 (2010)
Kumari, N., Dixit, M., Roesky, H.W., Mishra, L.: Chemistry for sustainable development. In: Bhowon, M.G., Jhaumeer-Lauloo, S., Li, H., Wah, K., Ramasami, P. (eds.) Chapter 15, p. 231. Springer, New York (2012)
Sakaguchi, U., Addison, A.W.: Spectroscopic and redox studies of some copper(II) complexes with biomimetic donor atoms: implications for protein copper centres. J. Chem. Soc. Dalton Trans. 4, 600 (1979)
Caspar, J.V., Meyer, T.J.: Photochemistry of MLCT excited states. Effect of nonchromophoric ligand variations on photophysical properties in the series cis-Ru(bpy)2L22+. Inorg. Chem. 22, 2444 (1983)
Wolfe, A., Shimer, G.H., Meehan, T.: Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 26, 6392 (1987)
Benesi, H.A., Hildebrand, J.H.: A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 71, 2703 (1949)
Marcus, Y.: Ionic radii in aqueous solutions. Chem. Rev. 88, 1475 (1988)
Fery-Forgues, S., Le Bries, M.-T., Guette, J.-P., Valeur, B.: Ion-responsive fluorescent compounds. 1. Effect of cation binding on photophysical properties of benzoxazinone derivative linked to monoaza-15-crown-5. J. Phys. Chem. 92, 6233 (1988)
Misra, A., Shahid, M.: Chromo and fluorogenic properties of some azo-phenol derivatives and recognition of Hg2+ ion in aqueous medium by enhanced fluorescence. J. Phys. Chem. C 114, 16726 (2010)
Acknowledgments
Financial support from DAE-BRNS (Grant No. 2005/37/33/BRNS/1807 to L. M.), Mumbai is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dixit, N., Shahid, M., Misra, A. et al. A hetero-bimetallic(Cu–Ru) chromogenic and fluorogenic complex as receptor of soft metal ions. J Incl Phenom Macrocycl Chem 76, 125–132 (2013). https://doi.org/10.1007/s10847-012-0181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0181-1