Abstract
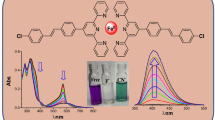
Two ratiometric fluorescence and colorimetric anion sensors were designed and synthesized according to simple Schiff base reaction. Two compounds 1 and 2 were characterized by ESI–MS, elemental analyses and 1H NMR. The sensors could give fast and visible color changes from yellow to red upon presence of the strong basic anions such as acetate ion. In particular, two compounds exhibited marked blue shifts (about 136 nm) in their emission spectra, when interacting with anions. Accordingly, the compounds 1 and 2 could act as real-time ratiometric fluorescence and colorimetric sensors for anions.









Similar content being viewed by others
References
Rebecca, M.D., Emma, B.V., Frederick, M.P., Paul, E.K., Thorfinnur, G.: Colorimetric and fluorescent anion sensors: an overview of recent developments in the use of 1,8-naphthalimide-based chemosensors. Chem. Soc. Rev. 39, 3936–3953 (2010)
Amendola, V., Fabbrizzi, L., Mosca, L.: Anion recognition by hydrogen bonding: urea-based receptors. Chem. Soc. Rev. 39, 3889–3915 (2010)
Moragues, M.E., Martínez-Máñez, R., Sancenón, F.: Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the year 2009. Chem. Soc. Rev. 40, 2593–2643 (2011)
Joseph, R., Rao, C.P.: Ion and molecular recognition by lower rim 1,3-di-conjugates of calix[4]arene as receptors. Chem. Rev. 111(8), 4658–4702 (2011)
Furman, P.A., Fyfe, J.A., Clair, M.H., Weinhold, K., Rideout, J.L., Freeman, G.A., Lehrman, S.N., Bolognesi, D.P., Broder, S., Mitsuya, H., Barry, D.W.: Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Nat. Acad. Sci. USA 83, 8333–8337 (1986)
Král, V., Sessler, J.L.: Molecular recognition via base-pairing and phosphate chelation. Ditopic and tritopic sapphyrin-based receptors for the recognition and transport of nucleotide monophosphates. Tetrahedron 51, 539–554 (1995)
Ojida, A., Mito-oka, Y., Sada, K., Hamachi, I.: Molecular recognition and fluorescence sensing of monophosphorylated peptides in aqueous solution by bis(zinc(II)-dipicolylamine)-based artificial receptors. J. Am. Chem. Soc. 126, 2454–2463 (2004)
Gale, P.A.: Amidopyrroles: from anion receptors to membrane transport agents. Chem. Commun. 30, 3761–3772 (2005)
Voet, D., Voet, J.G.: Biochemistry, 2nd edn. Wiley, New York (1995)
Ho, T.Y., Scranton, M.I., Taylor, G.T., Varela, R., Thunell, R.C., Muller-Karger, F.: Acetate cycling in the water column of the Cariaco Basin: seasonal and vertical variability and implication for carbon cycling acetate cycling in the water column of the Cariaco Basin: seasonal and vertical variability and implication for carbon cycling. Limnol. Oceanogr. 47, 1119–1128 (2002)
Galbraith, E., James, T.D.: Boron based anion receptors as sensors. Chem. Soc. Rev. 39, 3831–3842 (2010)
Wu, J.S., Liu, W.M., Ge, J.C., Zhang, H.Y., Wang, P.F.: New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 40, 3483–3495 (2011)
Zhou, Y., Yoon, J.: Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem. Soc. Rev. 41(1), 52–67 (2012)
Zhang, J.F., Zhou, Y., Yoon, J., Kim, J.S.: Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 40, 3416–3429 (2011)
Aragay, G., Pons, J., Merkoçi, A.: Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 111, 3433–3458 (2011)
Boens, N., Leen, V., Dehaen, W.: Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 41(3), 1130–1172 (2012)
Yu, H.B., Xiao, Y., Guo, H.Y., Qian, X.H.: Convenient and efficient fret platform featuring a rigid biphenyl spacer between rhodamine and BODIPY: transformation of ‘Turn-On’ sensors into ratiometric ones with dual emission. Chem. Eur. J. 17, 3179–3191 (2011)
Shao, J., Lin, H., Cai, Z.S., Lin, H.K.: A simple colorimetric and ON–OFF fluorescent chemosensor for biologically important anions based on amide moieties. J. Photochem. Photobiol. B 95, 1–5 (2009)
Shao, J., Lin, H., Lin, H.K.: Rational design of a colorimetric and ratiometric fluorescent chemosensor based on intramolecular charge transfer (ICT). Talanta 77, 273–277 (2008)
Wu, J.S., Zhou, J.H., Wang, P.F., Zhang, X.H., Wu, S.K.: New fluorescent chemosensor based on exciplex signaling mechanism. Org. Lett. 26, 2133–2136 (2005)
Lee, M.H., Quang, D.T., Jung, H.S., Yoon, J., Lee, C.H., Kim, J.S.: Ion-induced FRET on–off in fluorescent calix[4]arene. J. Org. Chem. 72, 4242–4245 (2007)
Jung, H.S., Kim, H.J., Vicens, J., Kim, J.S.: A new fluorescent chemosensor for F-based on inhibition of excited-state intramolecular proton transfer. Tetrahedron Lett. 50, 983–987 (2009)
Shao, J., Lin, H., Lin, H.K.: A simple and efficient colorimetric anion sensor based on a thiourea group in DMSO and DMSO–water and its real-life application. Talanta 75, 1015–1020 (2008)
Connors, K.A.: Binding constants, 1st edn. Wiley, New York (1987)
Helal, A., Kim, H.S.: Thiazole-based chemosensor III: synthesis and fluorescence sensing of CH3CO2 − based on inhibition of ESIPT. Tetrahedron 66, 7097–7103 (2010)
Helal, A., Thao, N.T.T., Lee, S.W., Kim, H.S.: Thiazole-based chemosensor II: synthesis and fluorescence sensing of fluoride ions based on inhibition of ESIPT. J. Incl. Phenom. Macrocycl. Chem. 66, 87–94 (2010)
Baggi, G., Boiocchi, M., Fabbrizzi, L., Mosca, L.: Moderate and advanced intramolecular proton transfer in urea–anion hydrogen-bonded complexes. Chem. Eur. J. 16, 9423–9439 (2011)
Acknowledgment
This work was supported by Natural Science Foundation of Universities of Inner Mongolia Autonomous Region (No: NG09168).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Shao, J. Ratiometric fluorescence and colorimetric sensing of anion utilizing simple Schiff base derivatives. J Incl Phenom Macrocycl Chem 76, 99–105 (2013). https://doi.org/10.1007/s10847-012-0177-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0177-x