Abstract
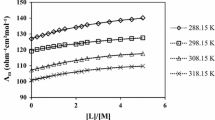
The complexation reaction between 4′,4″(5″)-di-tert-butyldibenzo-18-crown-6, ligand and Li+, Na+, K+, Mg2+, and Ba2+ ions were studied conductometrically in acetonitrile, ethanol, and methanol solutions. The formation constants of the 1:1 and 2:1 complexes (metal to ligand) were calculated from the computer fitting of the molar conductance in various mole ratios at 10, 20, 30, and 40 °C. The enthalpy and entropy changes of the complexation reactions in acetonitrile, ethanol, and methanol were estimated at four different temperatures.






Similar content being viewed by others
References
Pedersen, C.J.: Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89, 7017–7036 (1967)
Frensdorff, H.K.: Stability constants of cyclic polyether complexes with univalent cations. J. Am. Chem. Soc. 93, 600–606 (1971)
Pedersen, C.J., Frensdorff, H.K.: Macrocyclic polyethers and their complexes. Angew. Chem. Int. Ed. Engl. 11, 16–25 (1972)
Izatt, R.M., Bradshaw, J.S., Nielsen, S.A., Lamb, J.D., Christensen, J.J., Sen, D.: Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 85, 271–339 (1985)
Izatt, R.M., Pawlak, K., Bradshaw, J.S., Bruening, R.L.: Thermodynamic and kinetic data for macrocycle interactions with cations and anions. Chem. Rev. 91, 1721–2085 (1991)
Izatt, R.M., Pawlak, K., Bradshaw, J.S., Bruening, R.L.: Thermodynamic and kinetic data for macrocycle interaction with cations, anions, and neutral molecules. Chem. Rev. 95, 2529–2586 (1995)
Hasek, J., Huml, K., Hlavat, D.: The structure of a complex between rubidium thiocyanate, water and dibenzo[b,q][1,4,7,10,13,16,19,22,25,28]decaoxacyclotriacontane (dibenzo-30-crown-10). Acta Crystallagr. 35B, 330–334 (1975)
Buchanan, G.W., Lear, Y., Bensimon, C.: The sodium perchlorate complex of cis-syn-cis dicyclohexano-24-crown-8 ether. X-ray crystal structure and solution conformations as studied by variable temperature 13C NMR spectroscopy at 125 MHz. An example of eight-coordinate sodium. Can. J. Chem. 70, 1688–1695 (1992)
Live, D., Chan, S.I.: Nuclear magnetic resonance study of the solution structures of some crown ethers and their cation complexes. J. Am. Chem. Soc. 98, 3769–3778 (1976)
Shamsipur, M., Popov, A.I.: Multinuclear NMR study of dibenzo-30-crown-10 complexes with sodium, potassium, and cesium ions in nonaqueous solvents. J. Am. Chem. Soc. 101, 4051–4055 (1979)
Shamsipur, M., Rounaghi, G., Popov, A.I.: Sodium-23, cesium-133 and thallium-205 NMR study of sodium, cesium and thallium complexes with large crown ethers in nonaqueous solutions. J. Solution Chem. 9, 701–714 (1980)
Zollinger, D.P., Bulten, E., Christenhusz, A., Bos, M., Van Der Linden, W.: Computerized conductometric determination of stability constants of complexes of crown ethers with alkali metal salts and with neutral molecules in polar solvents. Anal. Chim. Acta 198, 207–222 (1987)
Wu, Y., Koch, W.: Absolute determination of electrolytic conductivity for primary standard KCl solutions from 0 to 50 °C. J. Solution Chem. 20, 391–401 (1991)
Shamsipur, M., Avanes, A., Aghapour, G., Sharghi, H.: Spectrophotometric studies of acidity constant and Cu2+ ion complexation of 1-hydroxy-2-(prop-2’-enyl)-4-( prop-2’-enyloxy)9-, 10-anthraquinone in methanol: water mixtures. Pol. J. Chem. 75, 1533–1541 (2001)
Gutmann, V.: The Donor-Acceptor Approach to Molecular Interactions. Plenum Press, New York (1978)
Katsuta, S., Ito, Y., Takeda, Y.: Stabilities in nitromethane of alkali metal ion complexes with dibenzo-18-crown-6 and dibenzo-24-crown-8 and their transfer from nitromethane to other polar solvents. Inorg. Chim. Acta 357, 541–547 (2004)
Christy, F.A., Shrivastav, P.S.: Conductometric studies on cation-crown ether complexes: a review. Crit. Rev. Anal. Chem. 41, 236–269 (2011)
Frisch, M., Trucks, G., Schlegel, H., Scuseria, G., Robb, M., Cheeseman, J., Montgomery Jr, J., Vreven, T., Kudin, K., Burant, J.: Revision, b 3 (2003)
Glendening, E.D., Feller, D.: An ab initio investigation of the structure and alkaline earth divalent cation selectivity of 18-crown-6. J. Am. Chem. Soc. 118, 6052–6059 (1996)
Ray, D., Feller, D., Michelle, B., Glendening, E.D., Armentrout, P.: Cation-ether complexes in the gas phase: bond dissociation energies and equilibrium structures of Li + (1, 2-dimethoxyethane) x, x = 1 and 2, and Li + (12-crown-4). J. Phys. Chem. 100, 16116–16125 (1996)
Sargent, A.L., Mosley, B.J., Sibert, J.W.: A theoretical investigation on the Wurster’s crown analogue of 18-crown-6. J. Phys. Chem. A 110, 3826–3837 (2006)
Masiker, M.C., Mayne, C.L., Boone, B.J., Orendt, A.M., Edward, M., Eyring, E.M.: 7Li NMR chemical shift titration and theoretical DFT calculation studies: solvent and anion effects on second-order complexation of 12-crown-4 and 1-aza-12-crown-4 with Lithium cation in several aprotic solvents. Magn. Reson. Chem. 48, 94–100 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Payehghadr, M., Heidari, R. Conductometric studies of the thermodynamics complexation of Li+, Na+, K+, Mg2+, and Ba2+ ions with 4′,4″(5″)-Di-tert-butyldibenzo-18-crown-6 ligand in acetonitrile, ethanol and methanol solutions. J Incl Phenom Macrocycl Chem 75, 205–210 (2013). https://doi.org/10.1007/s10847-012-0162-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0162-4