Abstract
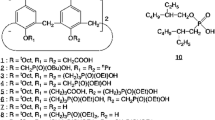
p-t-Octylcalix[4]arene with tetraphosphonic acid at lower rim in cone conformation has been designed and synthesized as a new extraction reagent to investigate the extraction behavior of the nine trivalent rare earth elements: La, Pr, Nd, Sm, Eu, Gd, Ho, Y, and Er. The extraction of rare earth metals with the present extractant occurs by a simple ion-exchange mechanism. The stoichiometry of the extractant to rare earth metal ion was determined to be 2:1 based on the extraction equation, half pH values, pH1/2, and the difference in the values of the extraction equilibrium constants of nine trivalent rare earth elements and separation factors between adjacent rare earth elements. This allowed for comparison of the estimated extraction efficiency and selectivity. The present extractant exhibited extremely high extractability and sufficiently high separation efficiency of rare earth metals, compared with calix[4]arene tetraphosphonic acid at upper rim, calix[4]arene tetraacetic acid at lower rim as previously reported and the commercial extraction reagent. This results was attributed to size and multidentate effects based on the preorganized cyclic structure of calix[4]arene and to the original selectivity of functional group for heavier rare earth elements.






Similar content being viewed by others
References
Rice, N.M.: Recent developments and potential uses for carboxylic acid extractants—a review. Hydrometallurgy 3, 111–133 (1978)
Preston, J.S.: Solvent extraction of metals by carboxylic acids. Hydrometallurgy 14(2), 171–188 (1985)
Freiser, H.: Solvent extraction of tervalent lanthanides as chelates—a systematic investigation of extraction equilibria. Solvent Extr. Ion Exch. 6(6), 1093–1108 (1988)
Cox, M.: New reagents. In: Alegret, S. (ed.) Developments in Solvent Extraction, Chap. 8, pp. 151–158. Ellis Horwood Ltd, Chichester (1988)
Otu, E.O., Westland, A.D.: Solvent extraction with organophosphonic mono-acidic esters. Solv. Extr. Ion Exch. 8(6), 759–781 (1990)
Yuan, C., Li, S., Feng, H.: In: Proceedings of ISEC’96, Value adding through Solvent Extraction, Melbourne, pp. 329–334 (1996)
Bogacki, M.B.: Physicochemical modification of copper extractants. A review. Solvent Extr Ion Exch 15(5), 731–755 (1997)
Kolarik, Z.: Recent trends in the search for new extractants. Min. Proc. Extr. Met. Rev Int. J. 21(1–5), 89–141 (2000)
Gloe, K., Stephan, H., Grotjahn, M.: Where is the Anion Extraction Going? Chem. Eng. Technol. 26(11), 1107–1117 (2003)
Cox, M.: Solvent extraction in hydrometallurgy. In: Rydberg, J., Cox, M., Musikas, C., Choppin, G.R. (eds.) Solvent extraction principles and practice, Chap. 11, pp. 455–505. Marcel Dekker, New York (2004)
Ritcey, G.M.: Extractants. In: Ritcey, G.M. (ed.) Solvent Extraction—Principles and Applications to Process Metallurgy vol 1, revised 2nd ed., Chap. 3, pp. 69–184. G.M. Ritcey & Associates Incorporated, Ottawa (2006)
Inoue, K., Nakashio, F.: Industrial chelate extractants—Preparation and recent progress. Chem. Eng. Jpn. 46(3), 164–171 (1982)
Sudderth, R.B., Kordosky, G.A.: Chemical processing. In: Malhotra, D., Riggs, W.F. (eds.) Some Practical Considerations in the Evaluation and Selection of Solvent Extraction Reagents, Chap. 20, pp. 181–196. S.M.E Littleton, Colorado (1986)
Ritcey, G.M.: Extractants. In: Ritcey, G.M. (ed.) Solvent extraction—Principles and Applications to Process Metallurgy volume 1, revised 2nd ed., Chap. 3, p. 70. G.M. Ritcey & Associates Incorporated, Ottawa (2006)
Peppard, D.F., Mason, G.W., Maier, J.L., Driscoll, W.J.: Fractional extraction of the lanthanides as their di-alkyl orthophosphates. J. Inorg. Nucl. Chem. 4, 334–343 (1957)
Peppard, D.F., Mason, G.W., Moline, S.W.: The use of dioctyl phosphoric acid extraction in the isolation of carrier-free 90Y, 140La, 144Ce, 143Pr, and 144Pr. J. Inorg. Nucl. Chem. 5, 141–146 (1957)
Peppard, D.F., Driscoll, W.J., Sironen, R.J., McCarry, S.: Nonmonotonic ordering of lanthanides in tributyl phosphate-nitric acid extraction systems. J. Inorg. Nucl. Chem. 4, 326–333 (1957)
Peppard, D.F., Mason, G.W., Hucher, I.: Stability constants of certain lanthanide(III) and actinide(III) chloride and nitrate complexes. J. Inorg. Nucl. Chem. 24, 881–888 (1962)
Peppard, D.F., Mason, G.W., Giffin, G.: Extraction of selected trivalent lanthanide and actinide cations by bis (hexoxy-ethyl)phosphoric acid. J. Inorg. Nucl. Chem. 27, 1683–1691 (1965)
Gutsche, C.D. (ed.): Calixarenes Revisited. Royal Society of Chemistry, Cambridge (1996)
Asfari, Z., Boehmer, V., Harrowfield, J.M., Vicens, J. (eds.): Calixarenes 2001. Kluwer, The Netherlands (2001)
Agrawal, Y.K., Kunji, S., Menon, S.K.: Analytical applications of calixarenes. Rev. Anal. Chem. 17(2), 69–139 (1998)
Izatt, R.M., Pawlak, K., Bradshaw, J.M.: Thermodynamic and kinetic data for macrocycle interactions with cations and anions. Chem. Rev. 91(8), 1721–1785 (1991)
Arnaud-Neu, F.: Solution chemistry of lanthanide macrocyclic complexes. Chem. Soc. Rev. 23(4), 235–241 (1994)
Roundhill, D.M.: Metal complexes of calixarenes. Prog. Inorg. Chem. 43, 533–592 (1995)
Ludwig, R.: Calixarenes in analytical and separation chemistry. Fresenius J. Anal. Chem. 367(2), 103–128 (2000)
Menon, S.K., Sewani, M.: Chemical modifications of calixarenes and their analytical applications. Rev. Anal. Chem. 25(1), 49–82 (2006)
Sliwa, W., Girek, T.: Calixarene complexes with metal ions. J. Incl. Phenom. Macrocycl. Chem. 66, 15–41 (2010)
Malone, J.F., Marrs, D.J., McKervey, M.A., O’Hagen, P., Thompson, N., Walker, A., Arnaud-Neu, F., Mauprivez, O., Weill, M.-J.S.: Calix[n]arene phosphine oxides. A new series of cation receptors for extraction of europium, thorium, plutonium and americium in nuclear waste treatment. J. Chem. Soc. Chem. Commun., 2151-2153 (1995)
Arnaud-Neu, F., Boehmer, V., Dozol, J.-F., Gruetter, C., Jakobi, R.A., Kraft, D., Mauprivez, O., Rouquette, H., Weill, M.-J.S., Simon, N., Vogt, W.: Calixarenes with diphenylphosphoryl acetamide functions at the upper rim. A new class of highly efficient extractants for lanthanides and actinides. J. Chem. Soc. Perkin Trans 2(6), 1175–1182 (1996)
Delmau, L.H., Simon, N., Weill, M.-J.S., Arnaud-Neu, F., Dozol, J.-F., Eymard, S., Tournois, B., Boehmer, V.: CMPO-substituted calix[4]arenes, extractants with selectivity among trivalent lanthanides and between trivalent actinides and lanthanides. Chem. Commun. 16, 1627–1628 (1998)
Delmau, L.H., Simon, N., Weill, M.-J.S., Arnaud-Neu, F., Dozol, J.-F., Eymard, S., Tuurnois, B., Gruetter, C., Musigmann, C., Tunayar, A., Boehmer, V.: Extraction of trivalent lanthanides and actinides by “CMPO-like” calixarenes. Sep. Sci. Technol. 34(6,7), 863–876 (1999)
Matthews, S.E., Saadioui, M., Boehmer, V., Barboso, S., Arnaud-Neu, F., Weill, M.-J.S., Jose, C.M., Alejandro, G., Dozol, J.-F.: Conformationally mobile wide rim carbamoylmethylphosphine oxide (CMPO)-calixarenes. J. Prakt. Chem. 341(3), 264–273 (1999)
Arnaud-Neu, F., Browne, J.K., Byrne, D., Marrs, D.J., McKervey, M.A., O’Hagen, P., Weill, M.-J.S., Walker, A.: Extraction and complexation of alkali, alkaline earth, and F-element cations by calixaryl phosphine oxides. Chem. Eur. J. 5(1), 175–186 (1999)
Barboso, S., Carrera, A.G., Matthews, S.E., Arnaud-Neu, F., Boehmer, V., Dozol, J.-F., Rouquette, H., Weill, M.J.-S.: Calix[4]arenes with CMPO functions at the narrow rim. Synthesis and extraction properties. J. Chem. Soc. Perkin Trans 2(4), 719–724 (1999)
Amatas, L., Klimchuk, O., Rudzevich, V., Pirozhenko, V., Kalchenko, V., Smirnov, I., Babain, V., Efremova, T., Varnek, A., Wipff, G., Arnaud-Neu, F., Roch, M., Saadioui, M., Boehmer, V.: New organophosphorus calix[4]arene ionophores for trivalent lanthanide and actinide cations. J. Supramol. Chem. 2(4–5), 421–427 (2003)
Schmidt, C., Saadioui, M., Boehmer, V., Host, V., Spirlet, M.-R., Desreux, J.F., Brisach, F., Arnaud-Neu, F., Dozol, J.-F.: Modification of calix[4]arenes with CMPO-functions at the wide rim. Synthesis, solution behavior, and separation of actinides from lanthanides. Org. Biomol. Chem. 1(22), 4089–4096 (2003)
Karavan, M., Arnaud-Neu, F., Hubscher-Bruder, V., Smirnov, I., Kalchenko, V.: Novel phosphophorylated calixarenes for the recognition of f-elements. J. Incl. Phenom. Macrocycl. Chem. 66, 113–123 (2010)
Harrowfield, J.M., Mocerino, M., Peachey, B.J., Skelton, B.W., White, A.H.: Rare-earth-metal solvent extraction with calixarene phosphates. J. Chem. Soc. Dalton Trans., 1687–1699 (1996)
Ludwig, R., Inoue, K., Yamato, T.: Solvent extraction behaviour of calixarene-type cyclophanes towards trivalent lanthanum, neodymium, europium, erbium, and ytterbium. Solvent Extr. Ion Exch. 11(2), 311–330 (1993)
Ludwig, R.; Inoue, K.; Shinkai, S.; Gloe, K.; Solvent extraction behaviour of p-tert-butylcalix[n]arene carboxylic acid derivatives towards trivalent lanthanides and sodium. In: Proc.ISEC’93, Solvent Extraction in the Process Industries, vol. 1, pp. 273-278 (1993)
Ohto, K.; Yano, M.; Inoue, K.; Yamamoto, T.; Goto, M.; Nakashio, F.; Nagasaki, T.; Shinkai, S.: Extraction of Rare Earths with New Extractants of Calixarene Derivatives. In: Proc.ISEC’93, Solvent Extraction in the Process Industries, vol. 1, pp. 364–369 (1993)
Ohto, K., Yano, M., Inoue, K., Yamamoto, T., Goto, M., Nakashio, F., Shinkai, S., Nagasaki, T.: Solvent extraction of trivalent rare earth metal ions with carboxylate derivatives of calixarenes. Anal. Sci. 11(6), 893–902 (1995)
Ludwig, R.; Gauglitz, R.: Calixarene type extractants for metal ions with improved properties. In: Proc.ISEC’96. Value Adding Through Solvent Extraction, vol.1, pp. 365–369 (1996)
Ludwig, R., Kunogi, K., Dung, N., Tachimori, S.: A calixarene-based extractant with selectivity for AmIII over LnIII. Chem.Commun. 20, 1985–1986 (1997)
Ludwig, R., Lentz, D., Nguyen, T.K.D.: Trivalent lanthanide and actinide extraction by calixarenes with different ring sizes and different molecular flexibility. Radiochim. Acta 88(6), 335–343 (2000)
Oshima, T., Yamamoto, T., Ohto, K., Goto, M., Nakashio, F., Furusaki, S.: A Calixarene-based Phosphoric Acid Extractant for Rare Earth Separation. Solvent Extr. Res. Dev., Jpn. 8, 194–204 (2001)
Jurecka, P., Vojtisek, P., Novotny, K., Rohovec, J., Lukes, I.: Synthesis, characterization and extraction behaviour of calix[4]arene-based phosphonic acids. J. Chem. Soc. Perkin Trans 2(7), 1370–1377 (2002)
Matulkova, I., Rohovec, J.: Synthesis, characterization and extraction behaviour of calix[4]arene with four propylene phosphonic acid groups on the lower rim. Polyhedron 24, 311–317 (2005)
Ohto, K., Ota, H., Inoue, K.: Solvent extraction of rare earths with a calix[4]arene compound containing phosphonate groups introduced onto upper rim. Solvent Extr. Res. Dev. Jpn. 4, 167–182 (1997)
Ohto, K., Yamasaki, T., Inoue, K.: Extractive separation of rare earth ions by using calix[4]arene with isopropyl hydrogen phosphonate at upper rim. Ars Sep. Acta 4, 96–106 (2007)
Ford-Moore, A.H., Williams, J.H.: The reaction between trialkyl phosphites and alkyl halides. J. Chem. Soc. 69, 1465–1467 (1947)
Harned, H.S., Owen, B.B. (eds.): The Physical Chemistry of Electrolytic, 3rd edn, p. 748. Reinhold Publishing Corporation, New York (1958)
Eigen, M.: Fast elementary steps in chemical reaction mechanisms. Pure Appl. Chem. 6(1), 97–115 (1963)
Kolarik, Z., Pankova, H.: Acidic organophosphorus extractants—I Extraction of lanthanides by means of dialkyl phosphoric acids-effect of structure and size of alkyl group. J. Inorg. Nucl. Chem. 28, 2325–2333 (1966)
Peppard, D.F., Mason, G.W., Lewey, S.: A tetrad effect in the liquid–liquid extraction ordering of lanthanides (III). J. Inorg. Nucl. Chem. 31, 2271–2272 (1960)
Author information
Authors and Affiliations
Corresponding author
Additional information
This article paper is dedicated to Prof. Leonard F. Lindoy on the celebration of his 75th birthday.
Rights and permissions
About this article
Cite this article
Ohto, K., Matsufuji, T., Yoneyama, T. et al. Preorganized, cone-conformational calix[4]arene possessing four propylenephosphonic acids with high extraction ability and separation efficiency for trivalent rare earth elements. J Incl Phenom Macrocycl Chem 71, 489–497 (2011). https://doi.org/10.1007/s10847-011-9998-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-9998-2