Abstract
Two isomeric polymacrocyclic ligands, L1 and L2 have been synthesized and the corresponding copper(II) complexes prepared. Reaction of the macrobicycle 15-oxa-1,5,8,12-tetraazabicyclo[10.5.2]nonadecane (L3) with diglycolyl chloride in base, yielded a diamide and following reduction, led to formation of the tricyclic ligand L1. A single crystal X-ray study of the blue, [Cu(L1)](ClO4)2, (C2/c monoclinic, a = 33.518(4), b = 16.3778(18), c = 13.7391(19) Å, β = 90.56(3)°, V = 7541.8 (16) Å3, Z = 12, R (F0) = 0.065, Rw = 0.066) reveals the presence of two independent [Cu(L1)]2+ cations displaying either five- or six- coordinate geometry. In the former distorted square pyramid, only one of the ether oxygens of the ligand is bound to the copper center, Cu–N1 = 2.083(7), Cu–N2 = 2.076(9), Cu–O1 = 2.276(11) Å and the Cu–O bond is at the longer end of axial distances of this type. However, the six-coordinate species is considerably more distorted, with in equivalence in both the metal-nitrogen and -oxygen bonds, Cu–N1 = 2.082(7), Cu–N2 = 2.096(7), Cu–N3 = 2.103(7), Cu–N4 = 2.068(7), Cu–O1 = 2.597(7), Cu–O2 = 2.427(7) Å, N(1)–Cu–O(1) = 71.0(2), N(3)–Cu–O(1) = 71.6(2), O(1)–Cu–O(2) = 64.6(2). The ligand L2 has been synthesized by reaction of the ten-membered macrocycle 1-oxa-4,8-diaza decane, (10-N2O) in dichloromethane with two moles of chloroacetyl chloride. The bis-pendant-armed product was further reacted with another mole of 10-N2O to yield a tricyclic diamide. The crystal structure of the intermediate diamide (Pnam, no.62 orthorhombic, a = 13.712(9), b = 9.111(5), c = 15.110(7) Å, V = 1887.6 Å3, Z = 8, R = 0.103, Rw = 0.103) has been determined. Subsequent reduction led to the formation of L2. The ligand is readily protonated to give a diammonium cation, [H2L2]2+. A single crystal structure of the [Cu(L2)](ClO4)2·CH3NO2 complex (P21/c, monoclinic a = 9.731(3), b = 16.065(5), c = 18.076(6) Å, β = 91.627(7)°, V = 2824.5 (15) Å3, Z = 4, R1 = 0.090, wR2 = 0.215) indicates considerable asymmetry in the cyclam plane (Cu–N1 = 2.099(11), Cu–N2 = 2.061(10), Cu–N3 = 2.065(10), Cu–N4 = 2.111(11), Cu-O12 = 2.410(7) Å) with one of the ether oxygens is coordinated to the metal, while the other is unbound. The ligand adopts a syn-configuration with both the attached macrocyclic units on the same side of the cyclam ring. Spectroscopic studies are reported.






Similar content being viewed by others
References
Lindoy, L.: Chemistry of Macrocyclic Ligand Complexes. Cambridge University Press, Cambridge (1989)
Fabrizzi, L.: The stabilization of high oxidation states of metals through coordination by poly-aza macrocycles. Comments Inorg. Chem. 4, 33–54 (1985)
Melson, G.A. (ed.): Coordination Chemistry of Macrocyclic Compounds. Plenum Press, New York (1979)
Joseph, E.B., Connolly, P.J., Sardella, D.J., Jasinski, J.P., Butcher, R.J.: Conformational characterization of square planar nickel(II) tetraaza macrocyclic complexes by proton NMR. Crystal structure of [Ni(13aneN4)]ZnCl4 Inorg. Chim. Acta. 230, 19–28 (1995)
Pierce, D.T., Hatfield, T.L., Billo, J., Ping, Y.: Oxidatively induced isomerization of square-planar [Ni(1, 4, 8, 11-tetraazacyclotetradecane)](ClO4)2. Inorg. Chem. 36, 2950–2955 (1997)
Barefield, K.E.: Coordination chemistry of the N-tetraalkylated cyclam ligands–a status report. Coord. Chem. Rev. 254, 1607–1627 (2010)
Bosnich, B., Poon, C.K., Tobe, M.L.: Complexes of cobalt(III) with a cyclic tetradentate secondary Amine. Inorg. Chem. 4, 1102–1108 (1965)
Fortier, D.G., McAuley, A.: Synthesis of a novel macrobicyclic ligand, 15-thia-1, 5, 8, 12- tetraazabicyclo[10.5.2]nonadecane, and its nickel(II) and copper(II) complexes. X-ray crystal structures of [Cu(L1)](ClO4)2 and [Ni(L1)(ClO4)]ClO4. Inorg. Chem. 28, 655–662 (1989)
Fortier, D.G., McAuley, A.: Template synthesis of the macrobicyclic ligand 1, 5, 8, 12, 15- pentaazabicyclo[10.5.2]nonadecane: evidence for imidate and enamine intermediates stabilized by copper(II). J. Am. Chem. Soc. 112, 2640–2647 (1990)
McAuley, A., Fortier, D.F., Macartney, D.H., Whitcombe, T.W.: Xu, C.: Synthesis and crystal structure of nickel(II) complexes of macrobicyclic ligands: identification and electron-transfer reactions of the corresponding nickel(III) complexes in solution. J. Chem. Soc., Dalton Trans. 1994(14), 2071–2079 (1994)
Beveridge, K.A., McAuley, A., Xu, C.: Preparation of the macrobicyclic ligand 15-oxa- 1, 5, 8, 12-tetraazabicyclo[10.5.2]nonadecane: characterization of copper(II) intermediates in a template synthesis. Inorg. Chem. 30, 2074–2082 (1991)
Ingham, A.M., Xu, C., Whitcombe, T.W., Bridson, J.N., McAuley, A.: Substitution reactions at the Ni(III) cation in macrobicyclic complexes–Reaction of metal ion complexes prepared from ligands L1 (17-oxa-1,4,8,11-tetraazabicyclo[6.5.6]nonadecane) and L2 (17-oxa-1,4,8,11-tetraazabicyclo[10.5.2]nonadecane) with chloride ion. Can. J. Chem. 80, 155–162 (2002)
Coulter, K.R., McAuley, A., Rettig, S.J.: Synthesis and characterization of the pentadentate macrobicyclic ligand, 14-thia-1, 4, 8, 11-tetraazabicyclo[9.5.3]nonadecane (L1) and its nickel(II) complexes. X-ray crystal structure of [Ni(L1)(ClO4)](ClO4)·2[Ni(L1)(OH2)](ClO4)2·6H2O. Can. J. Chem. 79, 930–937 (2001)
Rodopoulos, M., Rodopoulos, T., Bridson, J.N., Elding, L.-I., Rettig, S.J., McAuley, A.: Synthesis of 14-oxa-1, 4, 8, 11-tetraazabicyclo[9.5.3]nonadecane (L1) and a spectroscopic and structural study of [Ni(L1)(ClO4)](ClO4) and of the macrobicyclic precursor diamide complex, [Ni(HL2)](ClO4); chloride substitution kinetics of the corresponding [Ni(III)(L1)]3+ species. Inorg. Chem. 40, 2737–2742 (2001)
Ingham, A.I., Rodopoulos, M., Coulter, K.R., Rodopoulos, T., Subramanian, S., McAuley, A.: Synthesis, characterization and reactivity of some macrobicyclic and macrotricyclic hetero-clathrochelate complexes. Coord. Chem. Rev. 233–234, 255–271 (2002)
Bu, H., An, D.L., Cao, X.C., Zhang, R.H., Clifford, T., Kimura, E.: New dioxocyclam ligands appended with 2-pyridylmethyl pendant(s): synthesis, properties and crystal structure of their copper(II) complexes (dioxocyclam = 1,4,8,11-tetraazacyclotetradecane- 2,14-dione). J. Chem. Soc., Dalton. 1998(13), 2247–2252 (1998)
Barclay, T., McAuley, A., Subrananian, S.: Synthesis and structure of a new macrotricyclic ligand that encapsulates lithium and transition metal ions. J. Chem. Soc., Chem. Commun. 2002(2), 170–171 (2002)
Goeta, A.E., Howard, J.A.K., Maffeo, D., Puschmann, H., Williams, J.A.G., Yufit, D.S.: Copper(II) complexes of the isomeric tetraazamacrocyclic ligands 1,11- and 1,8-bis(2- pyridylmethyl)-1,4,8,11-tetraazacyclotetradecane and of the 1,4,8,11-tetraazacyclotetradecane-5,12-dione analogue at neutral and basic pH. J. Chem. Soc, Dalton, Soc. 2000(12), 1873–1880 (2000)
Luo, H., Rogers, R.D., Brechbiel, M.W.: A convenient and selective route to a trans- difunctionalized macrocyclic hexadentate N4O2 ligand. Can. J. Chem. 79, 1105–1109 (2001)
McAuley, A., Subramanian, S., Barclay, T.: Synthesis, structure and electrochemistry of isomeric nickel(II) complexes of a [9]ane fused cyclam macrotricycle: evidence for a stable trans-IV and a redox induced rearrangement in a trans-I conformation. J. Chem. Soc. Dalton 39, 9956–9961 (2010)
Weisman, G.R., Rogers, M.R., Wong, E.H., Jasinski, J.P., Paight, E.S.J.: Cross-bridged cyclam. Protonation and lithium cation (Li+) complexation in a diamond-lattice cleft. J. Am. Chem. Soc. 112, 8604–8605 (1990)
Coppens, P., Lieserowich, L., Rabinovich, D. modified by Bushnell, G.W.: unpublished
Sheldrick, G.M.: SHELX-76, Programs for Crystal Structure Solution and Refinement. University of Cambridge, Cambridge (1976)
Gabe, E.J., Le Page, Y., Charland, J.-P., Lee, F.L., White, P.S.: NRCVAX–an interactive program system for structure analysis. J. Appl. Cryst. 22, 384–387 (1989)
Scattering Factors from Int. Tab. Vol. 4: International Tables for X-ray Crystallography, Vol. IV. Kynoch Press, Birmingham (1974)
Johnson, C.K.: ORTEP—A Fortran Thermal Ellipsoid Plot Program. Technical Report ORNL-5138, Oak Ridge (1976)
Sheldrick, G.M.: SADABS. University of Göttingen, Germany (1996)
Sheldrick, G.M.: SHELX-97, Programs for Crystal Structure Solution and Refinement. University of Göttingen, Germany (1997)
Ingham, A.I., Xu, C.: unpublished observations
Tomlinson, A.A.G., Hathaway, B.M.: The electronic properties and stereochemistry of the copper(II) ion. Part II. The monoamine adducts of bisethylenediaminecopper(II) complexes. J. Chem. Soc. (A). 1968, 1685–1688 (1968)
Springborg, J., Kofod, P., Olsen, C.E., Toftlund, H., Søtofte, I.: Synthesis and crystal structure of a small bicyclic tetra-aza- proton sponge 1,4,7,10-tetra-aza[5.5.3] pentadecane dibromide perchlorate. Acta Chim. Scand. 49, 547–554 (1995)
Alder, R.W., Moss, R.E., Sessions, R.B.: Intrabridgehead hydrogen-bonded ions: spectroscopic characteristics and the question of single vs double minimum potentials. J. Chem. Soc. Chem. Comm. 997, 1000–1002 (1983)
Bencini, A., Bianchi, A., Bazzicalupi, C., Ciampolini, M., Fusu, V., Micheloni, M., Nardi, N., Paoli, P., Valtancoli, B.: Proton inclusion properties of a new azamacrocycle. Synthesis, characterization and crystal structure of [H3L][Cl]3·2H2O (L = 4, 10-dimethyl-1,4,7,10-tetraazabicyclo [5.5.2] tetradecane). Supramol. Chem. 3, 141–146 (1994)
Nave, C., Truter, M.: Crystal structure of the dihydroperchlorate of 1, 4, 8, 11-tetra- azacyclotetradecane (cyclam). J. Chem. Soc. Dalton Trans. 21, 2351–2354 (1974)
McAuley, A., Subramanian, S.: Formation of multinuclear complexes: new developments from cyclam derivatives. Coord. Chem. Rev. 200–202, 75–103 (2000)
Subramanian, S., Barclay, T.M., Coulter, K.R., McAuley, A.: Synthesis and characterization of polymacrocyclic ligands and protonated ions. Coord. Chem. Rev. 245, 65–71 (2003)
Acknowledgments
We wish to thank NSERC and the University of Victoria for support of this work. We also acknowledge the contribution of Mrs. K. Beveridge in the solution of the crystal structure of amide L6.
Author information
Authors and Affiliations
Corresponding author
Additional information
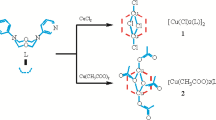
Two new isomeric macrotricyclic ligands (L1) and (L2) have been prepared. L1 was prepared by reaction of the corresponding tetraazamacrobicycle with diglycolyl chloride in base, followed by reduction. Whereas, L2 was prepared by coupling of [10]aneN2O with chloroacetyl chloride in two steps followed by reduction. Crystals of the copper(II) complex of L1 showed the presence of two species within the unit cell: a) a five-coordinate ion with one of the [10]aneN2O units uncoordinated, and b) the distorted six-coordinate Cu(II) ion shown. The isomeric ion, [Cu(L2)]2+, based on bridging across the 1,11 and 4,8-N donors of the cyclam ring, shows only the five-coordinate form.
It is a pleasure to recognize the contributions to macrocyclic chemistry made by friend and colleague Len Lindoy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10847_2011_9997_MOESM1_ESM.pdf
Crystal structure data (experimental, structure analysis parameters and methods, full list of bond length and angles), graphs showing statistical distribution of Cu–O distances for the 4-, 5- and 6-coordinate copper(II) complexes collected from Cambridge Structure Data base are available as supplementary materials. CIF file (CCDC 823433) for [Cu(L2)](ClO4)2·CH3NO2 is available from the Cambridge Data base. (PDF 1651 kb)
Rights and permissions
About this article
Cite this article
Ingham, A.I., McAuley, A., Subramanian, S. et al. Synthesis of the macrotricyclic ligands 8,18 dioxa-1,5,11,15-tetraaza-[13.5.2.25,11]-eicosane (L1) and 7,16 dioxa-1,4,10,13-tetraaza-[11.5.3.34,10]-octadecane (L2). Crystal structures of the copper(II) complexes, [Cu(L1)](ClO4)2 and [Cu(L2)](ClO4)2·CH3NO2 . J Incl Phenom Macrocycl Chem 71, 445–453 (2011). https://doi.org/10.1007/s10847-011-9997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-9997-3