Abstract
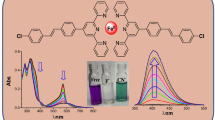
A novel fluorescent anion sensor 1 based on boradiazaindacenes (BODIPY) derivative was synthesized and its absorption and fluorescence properties were investigated in various solvents. 1 exhibited a red shift of absorption spectrum and fluorescence quenching in varying degree in the presence of F−, AcO−, H2PO4 − and Cl− due to multiple hydrogen bonding interactions between these anions and calix[4]pyrrole receptor. As an anion sensor in the visible region, 1 displayed the similar selectivity and sensitivity toward anions compared to the parent calix[4]pyrrole. However, 1 can be used as an effective dual responsive optical sensor for F− via chromogenical and fluorogenical signals.








Similar content being viewed by others
References
Gale, P.A., Garcia-Garrido, S.E., Garric, J.: Anion receptors based on organic frameworks: highlights from 2005 and 2006. Chem. Soc. Rev. 37, 151–190 (2008)
Steed, J.W.: Coordination and organometallic compounds as anion receptors and sensors. Chem. Soc. Rev. 38, 506–519 (2009)
Caltagirone, C., Gale, P.A.: Anion receptor chemistry: highlights from 2007. Chem. Soc. Rev. 38, 520–563 (2009)
Kubik, S.: Amino acid containing anion receptors. Chem. Soc. Rev. 38, 585–605 (2009)
Amendola, V., Fabbrizzi, L.: Anion receptors that contain metals as structural units. Chem. Commun. 5, 513–531 (2009)
Loudet, A., Burgess, K.: BODIPY dyes and their derivatives: synthesis and spectroscopic properties. Chem. Rev. 107, 4891–4932 (2007) and references therein
Ulrich, G., Ziessel, R., Harriman, A.: The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew. Chem. Int. Ed. 47, 1184–1201 (2008)
Coskun, A., Baytekin, B.T., Akkaya, E.U.: Novel fluorescent chemosensor for anions via modulation of oxidative PET: a remarkable 25-fole enhancement of emission. Terahedron Lett. 44, 5649–5651 (2003)
Ekmekci, Z., Yilmaz, M.D., Akkaya, E.U.: A monostyryl-boradiazaindacene (BODIPY) derivative as colorimetric and fluorescent probe for cyanide ions. Org. Lett. 10, 461–464 (2008)
Shiraishi, Y., Maehhara, H., Sugii, T., Wang, D., Hirai, T.: A BODIPY-indole conjugate as a colorimetric and fluorometric probe for selective fluoride anion detection. Tetrahedron Lett. 50, 4293–4296 (2009)
Gale, P.A., Sessler, J.L., Král, V.: Calixpyrroles. Chem. Commun. 1, 1–8 (1998)
Gale, P.A., Anzenbacher, J.P., Sessler, J.L.: Calix[4]pyrroles II. Coordination Chem.Rev. 222, 57–102 (2001) and references therein
Lee, C.H., Miyaji, H., Yoon, D.W., Sessler, J.L.: Strapped and other topographically nonplanar calixpyrrole analogues. Improved anion receptors. Chem. Commun. 1, 24–34 (2008)
Miyaji, H., Anzenbacher, P.J., Sessler, J.L., Bleasdale, E.R., Gale, P.A.: Anthracene-linked calix[4]pyrroles: fluorescent chemosensors for anions. Chem. Commun. 17, 1723–1724 (1999)
Anzenbacher, J.P., Jursíková, K., Sessler, J.L.: Second generation calixpyrrole anion sensors. J. Am. Chem. Soc. 122, 9350–9351 (2000)
Miyaji, H., Sato, W., Sessler, J.L.: Naked-eye detection of anions in dichloromethane: colorimetric anion sensors based on calix[4]pyrrole. Angew. Chem. Int. Ed. 39, 1777–1780 (2000)
Zhang, X., Xiao, Y., Qian, X.: Highly efficient energy transfer in the light harvesting system composed of three kinds of boron-dipyrromethene derivatives. Org. Lett. 10, 29–32 (2008)
Nishiyabu, R., Anzenbacher, J.P.: 1,3-Indane-based chromogenic calixpyrroles with push–pull chromophores: synthesis and anion sensing. Org. Lett. 8, 359–362 (2006)
Kollmannsberger, M., Rurack, K., Resch-Genger, U., Daub, J.: Ultrafast charge transfer in amino-substituted boron dipyrromethene dyes and its inhibition by cation complexation: a new design concept for highly sensitive fluorescent probes. J. Phys. Chem. A 102, 10211–10220 (1998)
Sunahara, H., Urano, Y., Kojima, H., Nagano, T.: Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing photoinduced electron-transfer-controlled fluorescence ON/OFF switching. J. Am. Chem. Soc. 129, 5597–5604 (2007)
Rurack, K., Kollmannsberger, M., Daub, J.: Molecular switching in the near infrared (NIR) with a functionalized boron-dipyrromethene dye. Angew. Chem. Int. Ed. 40, 385–387 (2001)
Yuan, M., Li, Y., Li, J., Li, C., Liu, X., Lv, J., Xu, J., Liu, H., Wang, S., Zhu, D.: A colorimetric and fluorometric dual-modal assay for mercury ion by a molecule. Org. Lett. 9, 2313–2316 (2007)
Miyaji, H., Sato, W., Sessler, J.L., Lynch, V.M.: A ‘building block’ approach to functionalized calix[4]pyrroles. Tetrahedron Lett. 41, 1369–1373 (2000)
Nishiyabu, R., Anzenbacher, P.J.: Sensing of antipyretic carboxylates by simple chromogenic calix[4]pyrroles. J. Am. Chem. Soc. 127, 8270–8271 (2005)
Coskun, A., Akkaya, E.U.: Ion sensing coupled to resonance energy transfer: a highly selective and sensitive ratiometric fluorescent chenosensor for Ag (I) by a modular approach. J. Am. Chem. Soc. 127, 10464–10465 (2005)
Baruah, M., Qin, W., Flors, C., Hofkens, J., Vallée, R.A.L., Beljonne, D., Auweraer, M.V., Borggraeve, W.M.D., Boens, N.: Solvent and pH dependent fluorescent properties of a dimethylaminostyryl borondipyyromethenen dye in solution. J. Phys. Chem. A 110, 5998–6009 (2006)
Casey, K.G., Quitevis, E.L.: Effect of solvent polarity on nonradiative processes in xanthene dyes: rhodamine b in normal alcohols. J. Phys. Chem. 92, 6590–6594 (1988)
Almonasy, N., Nepraš, M., Hyková, Š., Lyčka, A., Čermák, J., Dvořák, M., Michl, M.: The synthesis of N-derivatives of 3-aminoperylene and their absorption and fluorescence properties. Dyes Pigment. 82, 164–170 (2009)
Tian, M., Peng, X., Feng, F., Meng, S., Fan, J., Sun, S.: Fluorescent pH probes based on boron dipyrromethene dyes. Dyes Pigment. 81, 58–62 (2009)
Conners, K.A.: Binding Constants: The Measurement of Molecular Complex Stability. Wiley, New York (1987)
Gale, P.A., Sessler, J.L., Allen, W.E., Tvermoes, N.A., Lynch, V.: Calix[4]pyrroles: C-rim substitution and tenability of anion binding strength. Chem. Commun. 7, 665–666 (1997)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 20972170 to S-J. Shao), the open fund of State Key Laboratory of Oxo Synthesis & Selective Oxidation (Grant No. OSSO2008kjk6 to S-J. Shao) and by the Natural Science Foundation of Gansu province (No. 096RJ2A033 to Y. Guo).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
UV–vis titration of sensor 1 (1 × 10−5 M in CH3CN) upon the addition of AcO− from 0 to 2.08 × 10−4 M. Inset: Nonlinear curve fitting as a function of [AcO−]. (TIFF 1143 kb)
Fig. S2
UV–vis titration of sensor 1 (1 × 10−5 M in CH3CN) upon the addition of H2PO4 − from 0 to 3.2 × 10−4 M. Inset: Nonlinear curve fitting as a function of [H2PO4 −]. (TIFF 1152 kb)
Fig. S3
UV–vis titration of sensor 1 (1 × 10−5 M in CH3CN) upon the addition of Cl− from 0 to 1.12 × 10−3 M. Inset: Nonlinear curve fitting as a function of [Cl−]. (TIFF 1060 kb)
Fig. S4
Changes in the emission spectrum of 1 (5 × 10−6 M in CH3CN) upon the addition of AcO− from 0 to 3.2 × 10−4 M. Inset: Nonlinear curve fitting as a function of [AcO−] monitored at 626 nm. Excitation was at 587 nm. (TIFF 658 kb)
Fig. S5
Changes in the emission spectrum of 1 (5 × 10−6 M in CH3CN) upon the addition of H2PO4 − from 0 to 8.48 × 10−4 M. Inset: Nonlinear curve fitting as a function of [H2PO4 −] monitored at 626 nm. Excitation was at 587 nm. (TIFF 892 kb)
Fig. S6
Changes in the emission spectrum of 1 (5 × 10−6 M in CH3CN) upon the addition of Cl− from 0 to 1.52 × 10−3 M. Inset: Nonlinear curve fitting as a function of [Cl−] monitored at 626 nm. Excitation was at 587 nm. (TIFF 823 kb)
Rights and permissions
About this article
Cite this article
lv, Y., Xu, J., Guo, Y. et al. A novel colorimetric and fluorometric anion sensor based on BODIPY-calix[4]pyrrole conjugate. J Incl Phenom Macrocycl Chem 72, 95–101 (2012). https://doi.org/10.1007/s10847-011-9946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-9946-1