Abstract
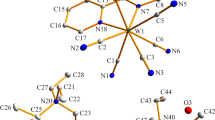
The selectivity, anion uptake and exchangeability of anion-binding by metal salt extractants of the form [M2 L 2]4+ have been assessed by the method of anion exchange chromatography in biphasic systems. The order of sulfate-, nitrate-, and chloride-uptake into the solid copper(II) complex as of the dioxime pro-ligand N,N′-dimethyl-N,N′-hexamethylenedi(3-hydroxyiminomethyl-2-hydroxy-5-tert-butylbenzylamine (L 1) is 56, 42, and 16%, respectively, consistent with the relative magnitudes of formation constants for the inclusion complexes, [A⊂Cu2 L 1 2]n+ where A = anion, found in UV–vis titration studies in a single phase. X-ray structural determination of the bis-benzylimine pro-ligand, N,N′-dimethyl-N,N′-hexamethylenedi-(3-benzyliminomehyl-2-hydroxy-5-tert-butylbenzylamine), nickel(II) sulfato complex [SO4⊂Ni2 L 2 2]SO4 reveals the nickel atoms to have a significant tetrahedral distortion, providing more favourable sulfate-alkylammonium interactions within the cage.





Similar content being viewed by others
Notes
Sample volume = 25 µL, Initial concentrations: SO4 (101.806 ppm), NO3 (127.969 ppm), Cl (74.192 ppm), m(SO4) = 101.806 mg/L * 25e−6 L = 2.545e−6 g, n(SO4) = 2.545e−6 g/96.064 g/mol = 2.649e−8 mol, 55.9% uptake, so n(SO4) taken up = 1.481e−8 mol, m(NO3) = 127.969 mg/L * 25e−6 L = 3.199e−6 g, n(NO3) = 3.199e−6/62.01 g/mol = 5.159e−8 mol, 42.3% uptake, so n(NO3) taken up = 2.182e−8 mol, m(Cl) = 74.192 mg/L * 25e−6 L = 1.855e−6 g, n(Cl) = 1.855e−6 g/35.453 g/mol = 5.232e−8 mol, 15.9% uptake, so n(Cl) taken up = 0.832e−8 mol, n(SO4):n(NO3 + Cl) = 1.481e−8 mol:3.014e−8 mol = 1:2.
1% HCO3 −/CO 2−3 buffer solution prepared from the eluent concentrate supplied by Dionex (P/N 063965).
References
Tasker, P.A., Plieger, P.G., West, L.C.: Metal complexes for hydrometallurgy and extraction. In: McCleverty, J.A., Meyer, T.B. (eds.) Comprehensive Coordination Chemistry II, vol. 5, pp. 759–808. Elsevier, Oxford (2004)
McDonald, R.G., Whittington, B.I.: Atmospheric acid leaching of nickel laterites review Part 1. Sulfuric acid technologies. Hydrometallurgy 91, 35–55 (2008)
Galbraith, S.G., L.F. Lindoy, P.A. Tasker, Plieger, P.G.: Simple procedures for assessing and exploiting the selectivity of anion extraction and transport. Dalton Trans. 1134–1136 (2006)
Galbraith, S.G., Henderson, D.K., Miller, H.A. Plieger, P.G., Tasker, P.A., Smith, K.J., West, L.C.: Development of extractants to transport metal salts in base metal recovery processes. Hydrometallurgy. In:Young, C. (ed.) Proceedings of 5th international symposium honoring Professor Ian M. Ritchie, Aug. 24–27. University of British Columbia, Vancouver, 1, pp. 941–954 (2003)
Akkus, N., Campbell, J.C., Davidson, J., Henderson, D.K., Miller, H.A., Parkin, A., Parsons, S., Plieger, P.G., Swart, R.M., Tasker, P.A., West, L.C.: Exploiting supramolecular chemistry in metal recovery: novel zwitterionic extractants for nickel(II) salts. Dalton Trans. 1932–1940 (2003)
Soldenhoff, K., Hayward, N., Wilkins, D.: Direct solvent extraction of cobalt and nickel from laterite-acid pressure leach liquors. EPD Congr. 1998, Proc. Sess. Symp. 153–165 (1998)
Gunn, D.M., Miller, H.A., Swart, R.M., Tasker, P.A., West, L.C., White, D.J.: New types of extractant to transport metal sulfates in base metal recovery processes. Int. Solvent Extr. Conf. 280–290 (2002)
Forgan, R.S., Davidson, J.E., Fabbiani, F.P.A., Galbraith, S.G., Henderson, D.K., Moggach, S.A., Parsons, S., Tasker, P.A., White, F.J.: Cation and anion selectivity of zwitterionic salicylaldoxime metal salt extractants. Dalton Trans. 39, 1763–1770 (2010)
Kordosky, G.A.: Copper recovery using leach/solvent extraction/electrowinning technology: forty years of innovation, 2.2 million tons of copper annually. Int. Solvent Extr. Conf. 853–862 (2002)
Mackey, P.J.: Rich heritage of copper production in Canada. CIM Magazine 2, 35–42 (2007)
Forgan, R.S., Roach, B.D., Wood, P.A., White, F.J., Campbell, J., Henderson, D.K., Kamenetzky, E., McAllister, F.E., Parsons, S., Pidcock, E., Richardson, P., Swart, R.M., Tasker, P.A.: Using the outer coordination sphere to tune the strength of metal extractants. Inorg. Chem. 50, 4515–4522 (2011)
Wenzel, M., Jameson, G.B., Ferguson, L.A., Knapp, Q.W., Forgan, R.S., White, F.J., Parsons, S., Tasker, P.A., Plieger, P.G.: Anion-induced contraction of helical receptors. Chem. Commun 28(24), 3606–3608 (2009)
Wenzel, M., Knapp, Q.W., Plieger, P.G.: A bis-salicylaldoximato-copper(II) receptor for selective sulfate uptake. Chem. Commun. 47, 499–501 (2011)
Plieger, P.G., Parsons, S., Parkin, A., Tasker, P.A.: Transport of metal salts; encapsulation of anions in dinuclear Cu(II) complexes [Cu2L2SO4]SO4 and [Cu2L2BF4](BF4)3, where L = 2,2′-[1,6-hexanediylbis[(methylimino)methylene]]bis[4-tert-butyl-6-(phenylazomethinyl)phenol]. J. Chem. Soc. Dalton Trans. 3928–3930 (2002)
Mercer, D.J., Loeb, S.J.: Metal-based anion receptors: an application of second-sphere coordination. Chem. Soc. Rev. 39, 3612–3620 (2010)
Kang, S.-O., Llinares, J.M., Day, V.W., Bowman-James, K.: Cryptand-like anion receptors. Chem. Soc. Rev. 39, 3980–4003 (2010)
Hay, B.P.: De novo structure-based design of anion receptors. Chem. Soc. Rev. 39, 3700–3708 (2010)
Kim, J.I., Juwarker, H., Liu, X., Lah, M.S., Jeong, K.S.: Selective sulfate binding induces helical folding of an indolocarbazole oligomer in solution and solid state. Chem. Commun. 46, 764–766 (2010)
Moyer, B.A., Delmau, L.H., Fowler, C.J., Ruas, A., Bostick, D.A., Sessler, J.L., Katayev, E., Pantos, G.D., Llinares, J.M., Hossain, A., Kang, S.O., Bowman-James, K.: Supramolecular chemistry of environmentally relevant anions. Adv. Inorg. Chem. 59, 175–204 (2007)
Eller, L.R., Stepien, M., Fowler, C.J., Lee, J.T., Sessler, J.L., Moyer, B.A.: Octamethyl-octaundecylcyclo[8]pyrrole: a promising sulfate anion extractant. J. Am. Chem. Soc. 129, 11020–11021 (2007)
Bowman-James, K.: Alfred Werner revisited: the coordination chemistry of anions. Acc. Chem. Res. 38, 671–678 (2005)
Ilioudis, C.A., Steed, J.W.: Organic macrocyclic polyamine-based receptors for anions. J. Supramol. Chem. 1, 165–187 (2003)
Choi, K., Hamilton, A.D.: Macrocyclic anion receptors based on directed hydrogen bonding interactions. Coord. Chem. Rev. 240, 101–110 (2003)
Amendola, V., Fabbrizzi, L., Mangano, C., Pallavicini, P., Poggi, A., Taglietti, A.: Anion recognition by dimetallic cryptates. Coord. Chem. Rev. 219–221, 821–837 (2001)
Gale, P.A.: Anion and ion-pair receptor chemistry: highlights from 2000 and 2001. Coord. Chem. Rev. 240, 191–221 (2003)
Gale, P.A., Quesada, R.: Anion coordination and anion-templated assembly: highlights from 2002 to 2004. Coord. Chem. Rev. 250, 3219–3244 (2006)
Mateus, P., Delgado, R., Brandao, P., Felix, V.: Polyaza cryptand receptor selective for dihydrogen phosphate. J. Org. Chem. 74, 8638–8646 (2009)
Wichmann, K., Antonioli, B., Soehnel, T., Wenzel, M., Gloe, K., Gloe, K., Price, J.R., Lindoy, L.F., Blake, A.J., Schroeder, M.: Polyamine-based anion receptors: extraction and structural studies. Coord. Chem. Rev. 250, 2987–3003 (2006)
Hennrich, G., Anslyn, E.V.: 1,3,5–2,4,6-functionalized, facially segregated benzenes-exploitation of sterically predisposed systems in supramolecular chemistry. Chem. Eur. J. 8, 2218–2224 (2002)
Filby, M.H., Steed, J.W.: A modular approach to organic, coordination complex and polymer based podand hosts for anions. Coord. Chem. Rev. 250, 3200–3218 (2006)
Wu, B., Liang, J., Yang, J., Jia, C., Yang, X.J., Zhang, H., Tang, N., Janiak, C.: Sulfate ion encapsulation in caged supramolecular structures assembled by second-sphere coordination. Chem. Commun. 15, 1762–1764 (2008)
Ravikumar, I., Lakshminarayanan, P.S., Arunachalam, M., Suresh, E., Ghosh, P.: Anion complexation of a pentafluorophenyl-substituted tripodal urea receptor in solution and the solid state: selectivity toward phosphate. Dalton Trans. 4160–4168 (2009)
Zhuge, F., Wu, B., Liang, J., Yang, J., Liu, Y., Jia, C., Janiak, C., Tang, N., Yang, X.-J.: Full- or half-encapsulation of sulfate anion by a tris(3-pyridylurea) receptor: effect of the secondary coordination sphere. Inorg. Chem. 48, 10249–10256 (2009)
Custelcean, R., Remy, P.: Selective crystallization of urea-functionalized capsules with tunable anion-binding cavities. Cryst. Growth Des. 9, 1985–1989 (2009)
Warr, R.J., Westra, A.N., Bell, K.J., Chartres, J., Ellis, R., Tong, C., Simmance, T.G., Gadzhieva, A., Blake, A.J., Tasker, P.A., Schroder, M.: Selective extraction and transport of the [PtCl6]2− anion through outer-sphere coordination chemistry. Chem. Eur. J. 15, 4836–4850 (2009)
Kumar, A., Sun, S–.S., Lees, A.J.: Directed assembly metallocyclic supramolecular systems for molecular recognition and chemical sensing. Coord. Chem. Rev. 252, 922–939 (2008)
Steed, J.W.: Coordination and organometallic compounds as anion receptors and sensors. Chem. Soc. Rev. 38, 506–519 (2009)
Mahoney Joseph, M., Nawaratna Gayathri, U., Beatty Alicia, M., Duggan Peter, J., Smith Bradley, D.: Transport of alkali halides through a liquid organic membrane containing a ditopic salt-binding receptor. Inorg Chem. 43, 5902–5907 (2004)
Mahoney Joseph, M., Beatty Alicia, M., Smith Bradley, D.: Selective solid-liquid extraction of lithium halide salts using a ditopic macrobicyclic receptor. Inorg. Chem. 43, 7617–7621 (2004)
Moyer, B.A., Bonnesen, P.V., Custelcean, R., Delmau, L.H., Hay, B.P.: Strategies for using host–guest chemistry in the extractive separations of ionic guests. Kem. Ind. 54, 65–87 (2005)
Holm, R.H., Swaminathan, K.: Nickel(II) complexes. IV. Bis(N-sec-alkylsalicylaldimine) complexes: conformational equilibriums in solution. Inorg. Chem. 2, 181–186 (1963)
Holm, R.H., Swaminathan, K.: Nickel(II) complexes III. Bis-(N-arylsalicylaldimine) complexes. Inorg. Chem. 1, 599–607 (1962)
Holm, R.H.: Nickel (II) complexes. II. The solution magnetism of bis(N-methylsalicylaldimine)nickel (II) and related complexes. J. Am. Chem. Soc. 83, 4683–4690 (1961)
Holm, R.H., McKinney, T.M.: Magnetic observations of some substituted Ni(II) salicylaldimine complexes. J. Am. Chem. Soc. 82, 5506–5507 (1960)
Holm, R.H.: Applications of isotropic shifts to the investigation of structures and structural equilibriums of metal complexes. Accounts Chem. Res. 2, 307–316 (1969)
Parkin, A., Barr, G., Collins, A., Dong, W., Gilmore, C.J., Tasker, P.A., Wilson, C.C.: Using cluster analysis to study transition-metal geometries: four-coordinate complexes with two salicylaldiminato or related ligands. Acta Crystallogr. B: Struct. Sci. B63, 612–620 (2007)
Akitsu, T.: Photofunctional supramolecular solution systems of chiral Schiff base nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes. Polyhedron 26, 2527–2535 (2007)
Akitsu, T., Einaga, Y.: Synthesis, crystal structures and electronic properties of Schiff base nickel(II) complexes: towards solvatochromism induced by a photochromic solute. Polyhedron. 24, 1869–1877 (2005)
Li, Z.-X., Zhang, X.-L., Pu, X.-H.: Bis[2-(benzyliminomethyl)-4-chlorophenolato-k2 N, O]nickel(II). Acta Crystallogr. E Struct. Rep. Online E64, m202/1–m202/7 (2008)
Chen, S.Z., Xia, D.G.: Bis{2-[(E)-benzyliminomethyl]-4-methylphenolato-k2 N, O}nickel(II). Acta Crystallogr. E Struct. Rep. Online. E65, m923 (2009)
Plieger, P.G., Tasker, P.A., Galbraith, S.G.: Zwitterionic macrocyclic metal sulfate extractants containing 3-dialkylaminomethylsalicylaldimine units. Dalton Trans. 313–318 (2004)
Sheldrick, G.M.: SHELXTL, 5.1. Bruker AXS, Madison (1998)
Van der Sluis, P., Spek, A.L.: BYPASS: an effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. A: Found. Crystallogr. A46, 194–201 (1990)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, J.YC., Parsons, S., Plieger, P.G. et al. Anion-selective receptors based on dinuclear copper(II) and nickel(II) cage complexes of bis-salicylaldimines. J Incl Phenom Macrocycl Chem 71, 529–536 (2011). https://doi.org/10.1007/s10847-011-0011-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-011-0011-x