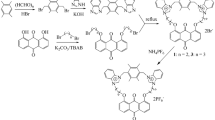
Abstract
Two new binaphthyl derivatives 1 and 2 bearing two imidazolium, or bisurea groups were investigated as fluorescent chemosensors for the chiral anion recognition. Receptors 1 and 2 displayed a better selectivity for (S)-2-phenylbutylate over (R)-2-phenylbutylate. Isothermal titration calorimeter methods, fluorescence as well as theoretical calculations are utilized to explain chiral selectivity.
Graphical Abstract






Similar content being viewed by others
References
Lee, C.-H., Miyaji, H., Yoon, D.-W., Sessler, J.L.: Strapped and other topographically nonplanar calixpyrrole analogues. Improved anion receptors. Chem. Commun. (1), 24–34 (2008)
Gale, P.A.: Structural and molecular recognition studies with acylic anion receptors. Acc. Chem. Res. 39, 465–475 (2006)
Yoon, J., Kim, S.K., Singh, N.J., Kim, K.S.: Imidazolium receptors for the recognition of anions. Chem. Soc. Rev. 35, 355–360 (2006)
Gunnlaugsson, T., Glynn, M., Tocci, G.M., Kruger, P.E., Pfeffer, F.M.: Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord. Chem. Rev. 250, 3094–3117 (2006)
Martínez-Máñez, R., Sancanón, F.: Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev. 103, 4419–4476 (2003)
Kim, S.K., Lee, D.H., Hong, J.-I., Yoon, J.: Chemosensors for pyrophosphate. Acc. Chem. Res. 42(1), 23–31 (2009)
Kim, S.K., Kim, H.N., Zhu, X., Lee, H.N., Lee, H.N., Soh, J.H., Swamy, K.M.K., Yoon, J.: Recent development of anion selective fluorescent chemosensors. Supramol. Chem. 19(4–5), 221–227 (2007)
Pu, L.: Fluorescence of organic molecules in chiral recognition. Chem. Rev. 104(3), 1687–1716 (2004). and references therein
Miyaji, H., Hong, S.-J., Jeong, S.-D., Yoon, D.-W., Na, H.-K., Hong, J., Ham, S., Sessler, J.L., Lee, C.-H.: A binol-strapped calix[4]pyrrole as a model chirogenic receptor for the enantioselective recognition of carboxylate anions. Angew. Chem. Int. Ed. 46(14), 2508–2511 (2007)
Kano, K., Nishiyabu, R.: General mechanism for chiral recognition by native and modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 44, 355–359 (2002)
Ragusa, A., Hayes, J.M., Light, M.E., Kilburn, J.D.: A combined computational and experimental approach for the analysis of the enantioselective potential of a new macrocyclic receptor for N-protected α-amino acids. Chem. Eur. J. 13(9), 2717–2728 (2007)
Jadhav, V.D., Schmidtchen, F.P.: Judging on host–guest binding mode uniqueness: association entropy as an indicator in enantioselection. Org. Lett. 8(11), 2329–2332 (2006)
Choi, M.K., Kim, H.N., Choi, H.J., Yoon, J., Hyun, M.H.: Chiral anion recognition by color change utilizing thiourea, azophenol and glucopyranosyl groups. Tetrahedron Lett. 49(29–30), 4522–4525 (2008)
Kameta, N., Nagawa, Y., Karikomi, M., Hiratani, K.: Chiral sensing for amino acid derivative based on a [2]rotaxane composed of an asymmetric rotor and an asymmetric axle. Chem. Commun. (35), 3714–3716 (2006)
Li, Z.-B., Lin, J., Pu, L.: A cyclohexyl-1, 2-diamine-derived bis(binaphthyl) macrocycle: enhanced sensitivity and enantioselectivity in the fluorescent recognition of mandelic acid. Angew. Chem. Int. Ed. 44(11), 1690–1693 (2005)
Kim, Y.K., Lee, H.N., Singh, N.J., Choi, H.J., Xue, J.Y., Kim, K.S., Yoon, J., Hyun, M.H.: Anthracene derivatives bearing thiourea and glucopyranosyl groups for the highly selective chiral recognition of amino acids: opposite chiral selectivities from similar binding units. J. Org. Chem. 73(1), 301–304 (2008)
Ryu, D., Park, E., Kim, D.-S., Yan, S., Lee, J.Y., Chang, B.-Y., Ahn, K.H.: A rational approach to fluorescence “turn-on” sensing of α-amino-carboxylates. J. Am. Chem. Soc. 130(8), 2394–2395 (2008)
Zhao, J., Davidson, M.G., Mahon, M.F., Kociok-Köhn, G., James, T.D.: An enantioselective fluorescent sensor for sugar acids. J. Am. Chem. Soc. 126(49), 16179–16186 (2004)
Kim, S.K., Kang, B.-G., Koh, H.S., Yoon, Y.-J., Jung, S.J., Jeong, B., Lee, K.-D., Yoon, J.: A new imidazolium cavitand for the recognition of dicarboxylates. Org. Lett. 6(25), 4655–4658 (2004)
Kwon, J.Y., Singh, N.J., Kim, H., Kim, S.K., Kim, K.S., Yoon, J.: Fluorescent GTP-sensing in aqueous solution of physiological pH. J. Am. Chem. Soc. 126(29), 8892–8893 (2004)
Kim, S.K., Singh, N.J., Kim, S.J., Kim, H.G., Kim, J.K., Lee, J.W., Kim, K.S., Yoon, J.: A new fluorescent photoinduced electron transfer chemosensor for the recognition of H2PO4 −. Org. Lett. 5(12), 2083–2086 (2003)
Xu, Z., Kim, S., Lee, K.-H., Yoon, J.: A highly selective fluorescent chemosensor for dihydrogen phosphate via unique excimer formation and PET mechanism. Tetrahedron Lett. 48(22), 3797–3800 (2007)
Kim, S.K., Singh, N.J., Kwon, J., Hwang, I.-C., Park, S.J., Kim, K.S., Yoon, J.: Fluorescent imidazolium receptors for the recognition of pyrophosphate. Tetrahedron 62(25), 6065–6072 (2006)
Lu, Q.-S., Dong, L., Zhang, J., Li, J., Jiang, L., Huang, Y., Qin, S., Hu, C.-W., Yu, X.-Q.: Imidazolium-functionalized BINOL as a multifunctional receptor for chromogenic and chiral anion recognition. Org. Lett. 11(3), 669–672 (2009)
Chianese, A.R., Crabtree, R.H.: Axially chiral bidentate N-heterocyclic carbene ligands derived from BINAM: rhodium and iridium complexes in asymmetric ketone hydrosilylation. Organometallics 24(13), 4432–4436 (2005)
Elstner, M., Porezag, D., Jungnickel, G., Elsner, J., Haugk, M., Frauenheim, T., Suhai, S., Seifert, G.: Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 58(11), 7260–7268 (1998)
Su, X., Luo, K., Xiang, Q., Lan, J., Xie, R.: Enantioselective recognitions of Chiral molecular tweezers containing imidazoliums for amino acids. Chirality 21(5), 539–546 (2009)
Acknowledgements
This work was supported by grants from KOSEF/MEST (SRC; R11-2005-008-02003-0) and collaboration fund between Korea and China (KOSEF; F01-2008-000-10026-0, Chinese NSFC; 50811140342). Authors also thank to Professor K. S. Kim and He Tian for helpful discussion. J.Y., S.K.K. and S-Y.C. thank to BK 21 fellowship. We also thank KBSI in Daegu, Korea, for their instrumentation assistance (FAB mass spectra).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swamy, K.M.K., Jiten Singh, N., Yoo, J. et al. Chiral binaphthyl receptors bearing imidazolium or urea groups for the recognition of anions. J Incl Phenom Macrocycl Chem 66, 107–111 (2010). https://doi.org/10.1007/s10847-009-9658-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9658-y