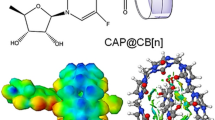
Abstract
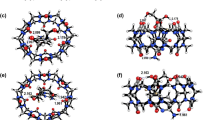
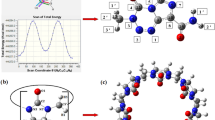
Geometries, formation and electronic properties of cucurbit[n]uril-oxaliplatin n = 5–8, host-guest complexes are investigated with DFT calculations. The formation of inclusion complexes of CB[n]-oxaliplatin are facile in CB[n] n = 6–8. In the complex, the cyclohexyl group is found to be deep inside the cavity, with the formation of a hydrogen bonding between the portal oxygen atoms and the amine nitrogen of the oxaliplatin guest. NBO analysis shows the transfer of charge from the metal center to the CB[7] unit and the existence of hydrogen bonding between the oxygen portal and amine nitrogen. The HOMO orbital is localized on the carboxylate group and the LUMO orbital are localized on the cucurbituril unit in CB[7]-oxaliplatin complex. The strength of the interaction determined here reflects the ability of CB[n] to act as a host for suitably oxaliplatin guests, even in aqueous solution.



Similar content being viewed by others
References
Sherman, S.E., Lippard, S.J.: Structural aspects of platinum anticancer drug interactions with DNA. Chem. Rev. 87, 1153–1181 (1987)
Lippert, B. (ed.): Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug. John Wiley and Sons Ltd, New York (1999)
Safra, T., Molho, R.B., Grisaru, D., Spigel, S., Geva, R., Matcejevsk, D., Inbar, M., Menzcer, J., Levy, T.: A phase-II study evaluating safety and efficacy with weekly paclitaxel and carboplatin as a primary treatment for patients with advanced epithelial ovarian cancer (EOC). J. Clin. Oncol. 24, 5077 (2006)
Chabner, B.A., Longo, D.L. (eds.): Cancer Chemotherapy and Biotherapy, Principles and Practice. Lippincott Williams and Wilkins (2006)
Ozols, R.F.: Ovarian cancer: is dose intensity dead. J. Clin. Oncol. 25, 4157–4158 (2007)
Li, X., Jasai, B.R. (eds.): Design of controlled release drug delivery systems. The Mc Graw-Hill companies (2006)
Krause-Heuer, A.M., Grant, M.P., Orkey, N., Aldrich-Wright, J.R.: Drug delivery devices and targeting agents for platinum (II) anticancer complexes. Aust. J. Chem. 61, 675–681 (2008)
Kemp, S., Wheate, N.J., Wang, S., Collins, J.G., Ralph, S.F., Day, A.I., Higgins, V.J., Aldrich-Wright, J.R.: Encapsulation of platinum(II)-based DNA intercalators within cucurbit[6, 7, 8]urils. J. Biol. Inorg. Chem. 12, 969–979 (2007)
Lagona, J., Mukhopadhyay, P., Chakrabarti, S., Isaacs, L.: The cucurbit[n]uril family. Angew. Chem. Int. Ed. 44, 4844–4870 (2005)
Tian, Z.C., Ni, X.L., Xiao, X., Wu, F., Zhang, Y.Q., Zhu, Q.J., Xue, S.F., Tao, Z.: Interaction models of three alkyl substituted cucurbit[6]uril with a hydrochloride salt of 4, 4′-dipyridyl guest. J. Mol. Struct. 888, 48–54 (2008)
Wang, R., Yuan, L., Ihmels, H., Macartney, D.H.: Cucurbit[8]uril/cucurbit[7]uril controlled off/on fluorescence of the acridizinium and 9-aminoacridizinium cations in aqueous solution. Chem.—A Eur. J. 13, 6468–6473 (2007)
Li, L.S., Ge, Y.H., Huang, Z.H., Li, Y.P.: Study on the molecular recognition of perhydroxycucurbit[6]uril with methyl orange by spectroscopic methods. Specrosc. Spectr. Anal. 27, 1393–1397 (2007)
Wyman, I.W., Macartney, D.H.: Cucurbit[7]uril host–guest complexes with small polar organic guests in aqueous solution. Org. Biomol. Chem. 6, 1796–1801 (2008)
Wheate, N.J., Kumar, P.G.A., Torres, A.M., Aldrich-Wright, J.R., Price, W.S.: Examination of cucurbit[7]uril and its host–guest complexes by diffusion nuclear magnetic resonance. J. Phys. Chem. B. 112, 2311–2314 (2008)
Liu, S.M., Shukla, A.D., Gadde, S., Wagner, B.D., Kaifer, A.E., Isaacs, L.: Ternary complexes comprising cucurbit[10]uril, porphyrins and guests. Angew. Chem. Int. Ed. 47, 2657–2660 (2008)
Liu, J.X., Long, L.S., Huang, R.B., Zheng, L.S.: Interesting anion—inclusion behavior of cucurbit[5]uril and its lanthanide—capped molecular capsule. Inorg. Chem. 46, 10168–10173 (2007)
Freeman, W.A., Mock, W.L., Shih, N.Y.: Cucurbituril. J. Am. Chem. Soc. 103, 7367–7368 (1981)
Kim, J., Jung, I.-S., Kim, S.-Y., Lee, E., Kang, J.-K., Sakamoto, S., Yamaguchi, K., Kim, K.: New cucurbituril homologues: synthesis, isolation, characterization, and X-ray crystal structure of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 122, 540–541 (2000)
Wheate, N.J.: Improving platinum(II)-based anticancer drug delivery using cucurbit[n]urils. J. Inorg. Biochem. 102, 2060–2066 (2008)
Jeon, Y.J., Kim, S.-Y., Ko, Y.H., Sakamoto, S., Yamaguchi, K., Kim, K.: Novel molecular drug carrier: encapsulation of oxaliplatin in cucurbit[7]uril and its effects on stability and reactivity of the drug. Org. Biomol. Chem. 3, 2122–2125 (2005)
Marquez, C., Hudgins, R.R., Nau, W.M.: Mechanism of host–guest complexation by cucurbituril. J. Am. Chem. Soc. 126, 5806–5816 (2004)
Pichierri, F.: DFT study of cucurbit[n]uril, n = 5–10. J. Mol. Struct. (Theochem.) 765, 151–152 (2006)
Buschmann, H.-J., Wego, A., Zielesny, A., Schollmeyer, E.: Structure, electronic properties and NMR-shielding of cucurbit[n]urils. J. Incl. Phenom. Macrocycl. Chem. 54, 85–88 (2006)
Oh, K.S., Yoon, J., Kim, K.S.: Structural stabilities and self-assembly of cucurbit[n]uril (n = 4–7) and decamethylcucurbit[n]uril (n = 4–6): a theoretical study. J. Phys. Chem. B. 105, 9726–9731 (2001)
Bakovets, V.V., Masliy, A.N., Kuznetsov, A.M.: Formation thermodynamics of cucurbit[6]uril macrocycle molecules: a theory study. J. Phys. Chem. B. 112, 12010–12013 (2008)
Pinjari, R.V., Gejj, S.P.: Electronic, structure, molecular electrostatic potential and NMR chemical shifts in cucurbit[n]urils (n = 5–8), ferrocene and their complexes. J. Phys. Chem. A. 112, 12679–12686 (2008)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A. Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, revision D.01. Gaussian, Inc., Wallingford, CT (2004)
Reed, A.L., Curtiss, L.A., Weinhold, F.: Intermolecular interactions from a natural bond orbital, donor-acceptor view point. Chem. Rev. 88, 899–926 (1988)
Bushchmann, H.-J., Wego, A., Zielesny, A., Schollmeyer, E.: Structure, stability, electronic properties and NMR-shielding of the cucurbit[6]uril-spermine-complex. J. Incl. Phenom. Macrocycl. Chem. 54, 241–246 (2006)
Tyagi, P., Gahlot, P., Kakkar, R.: Structural aspects of the anti-cancer drug oxaliplatin: a combined theoretical and experimental study. Polyhedron 27, 3567–3574 (2008)
Acknowledgments
Grants-in-aid for Research Project; Promotion of Advanced Medical Technology (H18-Nano-001); Ministry of Health, Labour and Welfare of Japan is gratefully acknowledged. The authors thank the crew of the Center for Computational Materials Science at Institute for Materials Research, Tohoku University, for their continuous support of the HITACHI SR11000 supercomputing facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suvitha, A., Venkataramanan, N.S., Mizuseki, H. et al. Theoretical insights into the formation, structure, and electronic properties of anticancer oxaliplatin drug and cucurbit[n]urils n = 5 to 8. J Incl Phenom Macrocycl Chem 66, 213–218 (2010). https://doi.org/10.1007/s10847-009-9601-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-009-9601-2