Abstract
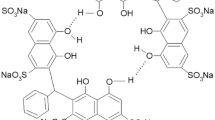
Disulfonated derivatives of 9,10-diphenyl anthracene (dsDPA) are known carriers of singlet oxygen. DsDPA and corresponding endoperoxides (dsDPAO2) form host–guest complexes with native cyclodextrins (i.e. β-CD and γ-CD). The modes of host–guest interaction were studied by 1H NMR and 2D-NMR (ROESY). Specific inclusions of phenyl groups of dsDPA/dsDPAO2 into the cyclodextrin cavities were found for both β-CD and γ-CD. The mode of interaction depends on the size of the CD cavity and the position of the sulfonate group.




Similar content being viewed by others
References
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1753 (1998)
Connors, K.A.: The stability of cyclodextrin complexes in solution. Chem. Rev. 97, 1325–1357 (1997)
Minns, J.W., Khan, A.: α-Cyclodextrin-I −3 host–guest complex in aqueous solution: theoretical and experimental studies. J. Phys. Chem. A 106, 6421–6425 (2002)
Uekama, K., Hirayama, F., Irie, T.: Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1198)
Singh, M., Sharma, R., Banerjee, U.C.: Biotechnological applications of cyclodextrins. Biotechnol. Adv. 20, 341–359 (2002)
Partyka, M., Ha Au, B., Evans, C.H.: Cyclodextrins as phototoxicity inhibitors in drug formulations: studies on model systems involving naproxen and β-cyclodextrin. J. Photochem. Photobiol. A 140, 67–74 (2001)
Schneider, H.J., Hacket, F., Rüdiger, V.: NMR studies of cyclodextrin and cyclodextrin complexes. Chem. Rev. 98, 1755–1785 (1998)
Djedaïne, F., Lin, S.Z., Perly, B., Wouessidjewe, D.: High-field nuclear-magnetic-resonance techniques for the investigation of a beta-cyclodextrin- indomethacin inclusion complex. J. Pharmacol. Sci.-US 79, 643–646 (1990)
Fielding, L.: Determination of association constants (K-a) from solution NMR data. Tetrahedron 56, 6151–6170 (2000)
Salvatierra, D., Jaime, C., Virgili, A., Sánchez-Ferrando, F.: Determination of the inclusion geometry for the β-cyclodextrin/benzoic acid complex by NMR and molecular modeling. J. Org. Chem. 61, 9578–9581 (1996)
Fernandes, C.M., Carvalho, R.A., Pereira da Costa, S., Veiga, F.J.B.: Multimodal molecular encapsulation of nicardipine hydrochloride by β-cyclodextrin, hydroxypropyl-β-cyclodextrin and triacetyl-β-cyclodextrin in solution. Structural studies by 1H NMR and ROESY experiments. Eur. J. Pharm. Sci. 18, 285–296 (2003)
Sun, D.-Z., Li, L., Qiu, X.-M., Liu, F., Yi, B.-L.: Isothermal titration calorimetry and H-1 NMR studies on host–guest interaction of paeonol and two of its isomers with beta-cyclodextrin. Int. J. Pharm. 316, 7–13 (2006)
Amato, M.E., Lipkowitz, K.B., Lombardo, G.M., Pappalardo, G.C.: High-field NMR spectroscopic techniques combined with molecular dynamics simulations for the study of the inclusion complexes of α- and β-cyclodextrins with the cognition activator 3-phenoxypyridine sulphate. Magn. Reson. Chem. 36, 693–705 (1998)
Cameron, K.S., Fletcher, D., Fielding, L.: An NMR study of cyclodextrin complexes of the steroidal neuromuscular blocker drug Rocuronium Bromide. Magn. Reson. Chem. 40, 251 (2002)
Schneider, H.J., Blatter, T., Simona, S.: NMR and fluorescence studies of cyclodextrin complexes with guest molecules containing both phenyl and naphthyl units. J. Am Chem. Soc. 113, 1996–2000 (1991)
Simona, S., Schneider, H.J.: NMR analyses of cyclodextrin complexes with substituted benzoic acid and benzoate anions. J. Chem. Soc. Perkin Trans. 2, 1717–1722 (2000)
Bothner-By, A.A., Stephens, R.L., Ju-mee Lee.: Structure determination of a tetrasaccharide: Transient nuclear overhauser effects in the rotating frame. J. Am. Chem. Soc. 106, 811–813 (1984)
Slavětínská, L., Mosinger, J., Kubát, P.: Supramolecular carriers of singlet oxygen: photosensitized formation and thermal decomposition of endoperoxides in the presence of cyclodextrins. J. Photochem. Photobiol. A (in press), doi: 10.1016/j.jphotochem.2007.09.007
Étienne, A., Lepeley, J.C., Heymés R.: Mémoires Présentés a la Société. Chimique 192, 835 (1949)
Aubry, J.M.: Search for singlet oxygen in the decomposition of hydrogen peroxide by mineral compounds in aqueous solutions. J. Am. Chem. Soc. 107, 5844–5849 (1985)
Aubry, J.M., Cazin, B.: Chemical sources of singlet oxygen. 2. Quantitative generation of singlet oxygen from hydrogen peroxide disproportionation catalyzed by molybdate ions. Inorg. Chem. 27, 2013–2014 (1988)
Nardello, V., Bogaert, S., Alsters, P.L., Aubry, J.M.: Singlet oxygen generation from H2O2/MoO 2−4 : peroxidation of hydrophobic substrates in pure organic solvents. Tetrahedron Lett. 43, 8731–8734 (2002)
Aubry, J.M., Cazin, B.: Chemical source of singlet oxygen. 3. Peroxidation of water-soluble singlet oxygen carriers with the hydrogen peroxide-molybdate systém. J. Org. Chem. 54, 726–728 (1989)
Hanessian, S., Benalil, A., Viet, M.T.P.: The intramolecular inclusion of aromatic esters within β-cyclodextrin as a function of chain length – a detailed NMR study. Tetrahedron 51, 10131–10148 (1995)
Forgo, P., D’Souza, V.T.: The application of slective ROE experiments to study solution structures of cyclomaltooligosacharide derivatives and complexes. Carbohydr. Res. 306, 473–478 (1998)
Acknowledgements
This work was supported by the Czech Science Foundation (Grants Nos. 203/08/0831, 203/07/1424 and 203/06/1244).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Jan Sejbal.
Rights and permissions
About this article
Cite this article
Slavětínská, L., Mosinger, J., Dračínský, M. et al. NMR study of host–guest complexes of disulfonated derivatives of 9, 10-diphenylanthracene and corresponding endoperoxides with cyclodextrins. J Incl Phenom Macrocycl Chem 61, 241–250 (2008). https://doi.org/10.1007/s10847-008-9416-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-008-9416-6